Ten Considerations for Integrating Patient-Reported Outcomes into Clinical Care for Childhood Cancer Survivors
- PMID: 36831370
- PMCID: PMC9954048
- DOI: 10.3390/cancers15041024
Ten Considerations for Integrating Patient-Reported Outcomes into Clinical Care for Childhood Cancer Survivors
Abstract
Patient-reported outcome measures (PROMs) are subjective assessments of health status or health-related quality of life. In childhood cancer survivors, PROMs can be used to evaluate the adverse effects of cancer treatment and guide cancer survivorship care. However, there are barriers to integrating PROMs into clinical practice, such as constraints in clinical validity, meaningful interpretation, and technology-enabled administration of the measures. This article discusses these barriers and proposes 10 important considerations for appropriate PROM integration into clinical care for choosing the right measure (considering the purpose of using a PROM, health profile vs. health preference approaches, measurement properties), ensuring survivors complete the PROMs (data collection method, data collection frequency, survivor capacity, self- vs. proxy reports), interpreting the results (scoring methods, clinical meaning and interpretability), and selecting a strategy for clinical response (integration into the clinical workflow). An example framework for integrating novel patient-reported outcome (PRO) data collection into the clinical workflow for childhood cancer survivorship care is also discussed. As we continuously improve the clinical validity of PROMs and address implementation barriers, routine PRO assessment and monitoring in pediatric cancer survivorship offer opportunities to facilitate clinical decision making and improve the quality of survivorship care.
Keywords: childhood cancer survivors; health-related quality of life; implementation; patient-reported outcomes; symptoms.
Conflict of interest statement
All co-authors declare no conflict of interest.
Figures
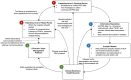
References
-
- Gibson T.M., Mostoufi-Moab S., Stratton K.L., Leisenring W.M., Barnea D., Chow E.J., Donaldson S.S., Howell R.M., Hudson M.M., Mahajan A., et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19:1590–1601. doi: 10.1016/S1470-2045(18)30537-0. - DOI - PMC - PubMed
-
- Bhakta N., Liu Q., Ness K.K., Baassiri M., Eissa H., Yeo F., Chemaitilly W., Ehrhardt M.J., Bass J., Bishop M.W., et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. - DOI - PMC - PubMed
-
- Varni J.W., Limbers C.A., Burwinkle T.M. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQLTM 4.0 Generic Core Scales. Health Qual. Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

