Ki67 as a Predictor of Response to PARP Inhibitors in Platinum Sensitive BRCA Wild Type Ovarian Cancer: The MITO 37 Retrospective Study
- PMID: 36831376
- PMCID: PMC9954459
- DOI: 10.3390/cancers15041032
Ki67 as a Predictor of Response to PARP Inhibitors in Platinum Sensitive BRCA Wild Type Ovarian Cancer: The MITO 37 Retrospective Study
Abstract
Background: There is compelling need for novel biomarkers to predict response to PARP inhibitors (PARPi) in BRCA wild-type (WT) ovarian cancer (OC).
Methods: MITO 37 is a multicenter retrospective study aiming at correlating Ki67 expression at diagnosis with a clinical outcome following platinum treatment and PARPi maintenance. Clinical data were collected from high grade serous or endometroid BRCAWT OC treated with niraparib or rucaparib maintenance between 2010-2021 in 15 centers. Ki67 expression was assessed locally by certified pathologists on formalin-fixed paraffin embedded (FFPE) tissues. Median Ki67 was used as a cut-off.
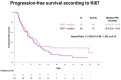
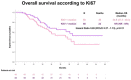
Results: A total of 136 patients were eligible and included in the analysis. Median Ki67 was 45.7% (range 1.0-99.9). The best response to platinum according to median Ki67 was 26.5% vs. 39.7% complete response (CR), 69.1% vs. 58.8% partial response (PR), 4.4% vs. 1.5% stable disease (SD). The best response to PARPi according to median Ki67 was 19.1% vs. 36.8% CR, 26.5% vs. 26.5% PR, 26.5 vs. 25% SD, 27.9% vs. 16.2% progressive disease (PD). No statistically significant differences in progression free survival (PFS) and overall survival (OS) were identified between low and high Ki67. PFS and OS are in line with registration trials.
Conclusions: Ki67 at diagnosis did not discriminate responders to PARPi.
Keywords: Ki67; PARP inhibitor; niraparib; ovarian cancer; rucaparib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Poveda A.M., Selle F., Hilpert F., Reuss A., Savarese A., Vergote I., Witteveen P., Bamias A., Scotto N., Mitchell L., et al. Bevacizumab Combined with Weekly Paclitaxel, Pegylated Liposomal Doxorubicin, or Topotecan in Platinum-Resistant Recurrent Ovarian Cancer: Analysis by Chemotherapy Cohort of the Randomized Phase III AURELIA Trial. J. Clin. Oncol. 2015;33:3836–3838. doi: 10.1200/JCO.2015.63.1408. - DOI - PubMed
-
- Penson R.T., Valencia R.V., Cibula D., Colombo N., Leath C.A., Bidziński M., Kim J.W., Nam J.H., Madry R., Hernández C., et al. Olaparib versus Nonplatinum Chemotherapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020;38:1164–1174. doi: 10.1200/JCO.19.02745. - DOI - PMC - PubMed
-
- Halverson J.L., Martinez-Donate A.P., Palta M., Leal T., Lubner S., Walsh M.C., Strickland J.S., Smith P.D., Trentham-Dietz A. Health Literacy and Health-Related Quality of Life Among a Population-Based Sample of Cancer Patients. J. Health Commun. 2015;20:1320–1329. doi: 10.1080/10810730.2015.1018638. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

