Practical Application of Circulating Tumor-Related DNA of Human Papillomavirus in Liquid Biopsy to Evaluate the Molecular Response in Patients with Oropharyngeal Cancer
- PMID: 36831390
- PMCID: PMC9953792
- DOI: 10.3390/cancers15041047
Practical Application of Circulating Tumor-Related DNA of Human Papillomavirus in Liquid Biopsy to Evaluate the Molecular Response in Patients with Oropharyngeal Cancer
Abstract
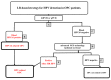
Recent findings have shown that human papillomavirus (HPV) DNA is present in the blood as a tumor-specific biomarker (circulating tumor-related HPV; ctHPV) in patients with HPV-related oropharyngeal cancer (HPV-related OPC). The molecular response (MR) in patients with HPV-related OPC can be defined as the change in the number of ctHPV copies in relation to its initial quantity. The optimal model for assessing the MR using a liquid biopsy (LB) should be based on the E6/E7 sequences of the viral genome. MR assessment can help to evaluate the intensity of ongoing treatments in relation to the tumor response. The evaluation of the residual disease at the end of therapy may also be performed by MR assessment. If a partial MR (pMR) is found, caution is indicated and a subsequent LB should be considered, due to the likelihood of disease progression. Complete radiological and clinical responses together with a complete MR (cMR) convincingly indicate a low risk of treatment failure. Moreover, molecular recurrence (Mrec) during a follow-up, confirmed in two consecutive assays, even despite the lack of any other clinical or radiological symptoms of progression, indicates patients at high risk of disease recurrence. In conclusion, MR by ctHPV assessment may hasten the early detection of disease progression, at any stage of the management of the patient with HPV-related OPC.
Keywords: chemotherapy; circulating tumor HPV DNA; human papillomavirus; liquid biopsy; molecular response; oropharyngeal cancer; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.D., Knippers R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. - PubMed
-
- Mouliere F., El Messaoudi S., Gongora C., Guedj A.S., Robert B., Del Rio M., Molina F., Lamy P.J., Lopez-Crapez E., Mathonnet M., et al. Circulating Cell-Free DNA from Colorectal Cancer Patients May Reveal High KRAS or BRAF Mutation Load. Transl. Oncol. 2013;6:319–328. doi: 10.1593/tlo.12445. - DOI - PMC - PubMed
-
- Diehl F., Li M., Dressman D., He Y., Shen D., Szabo S., Diaz L.A., Jr., Goodman S.N., David K.A., Juhl H., et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. - DOI - PMC - PubMed
-
- Anker P., Stroun M. Circulating DNA in plasma or serum. Medicina. 2000;60:699–702. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

