Immunotherapy in Melanoma: Recent Advances and Future Directions
- PMID: 36831449
- PMCID: PMC9954703
- DOI: 10.3390/cancers15041106
Immunotherapy in Melanoma: Recent Advances and Future Directions
Abstract
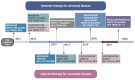
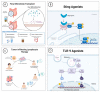
The use of immunotherapy in the treatment of advanced and high-risk melanoma has led to a striking improvement in outcomes. Although the incidence of melanoma has continued to rise, median survival has improved from approximately 6 months to nearly 6 years for patients with advanced inoperable stage IV disease. Recent understanding of the tumor microenvironment and its interplay with the immune system has led to the explosive development of novel immunotherapy treatments. Since the approval of the therapeutic cytokines interleukin-2 and interferon alfa-2 in the 1990s, the development of novel immune checkpoint inhibitors (ICIs), oncolytic virus therapy, and modulators of the tumor microenvironment have given way to a new era in melanoma treatment. Monoclonal antibodies directed at programmed cell death protein 1 receptor (PD-1) and its ligand (PDL-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and lymphocyte-activation gene 3 (LAG-3) have provided robust activation of the adaptive immune system, restoring immune surveillance leading to host tumor recognition and destruction. Multiple other immunomodulatory therapeutics are under investigation to overcome resistance to ICI therapy, including the toll-like receptor-9 (TLR-9) and 7/8 (TLR-7/8) agonists, stimulator of interferon genes (STING) agonists, and fecal microbiota transplantation. In this review, we focus on the recent advances in immunotherapy for the treatment of melanoma and provide an update on novel therapies currently under investigation.
Keywords: LAG-3; STING; T-VEC; TLR-9; adoptive cell therapy; fecal microbiota transplant; immune checkpoint blockade; immunotherapy; melanoma.
Conflict of interest statement
A.K. and L.K. declare no conflict of interest. J.M.K.—Consultant: Amgen, Inc., Bristol Myers Squibb, Checkmate, Immunocore LLC, Iovance Biotherapeutics, Istari Oncology, Merck, Novartis, OncoCyte Corporation, OncoSec Medical Inc., Pfizer, Regeneron, Replimune, Inc. Research Support: Amgen, Inc., Bristol Myers Squibb, Checkmate, Immunocore, LLC, Iovance, Novartis.
Figures
References
-
- Bedikian A.Y., Millward M., Pehamberger H., Conry R., Gore M., Trefzer U., Pavlick A.C., DeConti R., Hersh E.M., Hersey P. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J. Clin. Oncol. 2006;24:4738–4745. - PubMed
-
- Middleton M.R., Grob J., Aaronson N., Fierlbeck G., Tilgen W., Seiter S., Gore M., Aamdal S., Cebon J., Coates A. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000;18:158. doi: 10.1200/JCO.2000.18.1.158. - DOI - PubMed
-
- Korn E.L., Liu P.-Y., Lee S.J., Chapman J.-A.W., Niedzwiecki D., Suman V.J., Moon J., Sondak V.K., Atkins M.B., Eisenhauer E.A. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. - DOI - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021;39:9506. doi: 10.1200/JCO.2021.39.15_suppl.9506. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

