Relationship between Tumor Budding and Partial Epithelial-Mesenchymal Transition in Head and Neck Cancer
- PMID: 36831453
- PMCID: PMC9953904
- DOI: 10.3390/cancers15041111
Relationship between Tumor Budding and Partial Epithelial-Mesenchymal Transition in Head and Neck Cancer
Abstract
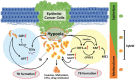
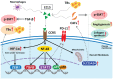
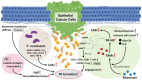
Tumor budding (TB), a microscopic finding in the stroma ahead of the invasive fronts of tumors, has been well investigated and reported as a prognostic marker in head and neck squamous cell carcinoma (HNSCC). Epithelial-mesenchymal transition (EMT) is a crucial step in tumor progression and metastasis, and its status cannot be distinguished from TB. The current understanding of partial EMT (p-EMT), the so-called halfway step of EMT, focuses on the tumor microenvironment (TME). Although this evidence has been investigated, the clinicopathological and biological relationship between TB and p-EMT remains debatable. At the invasion front, previous research suggested that cancer-associated fibroblasts (CAFs) are important for tumor progression, metastasis, p-EMT, and TB formation in the TME. Although there is biological evidence of TB drivers, no report has focused on their organized functional relationships. Understanding the mechanism of TB onset and the relationship between p-EMTs may facilitate the development of novel diagnostic and prognostic methods, and targeted therapies for the prevention of metastasis in epithelial cancer. Thus far, major pieces of evidence have been established from colorectal cancer (CRC), due to a large number of patients with the disease. Herein, we review the current understanding of p-EMT and TME dynamics and discuss the relationship between TB development and p-EMT, focusing on CAFs, hypoxia, tumor-associated macrophages, laminin-integrin crosstalk, membrane stiffness, enzymes, and viral infections in cancers, and clarify the gap of evidence between HNSCC and CRC.
Keywords: cancer-associated fibroblast; head and neck squamous cell carcinoma; partial epithelial–mesenchymal transition; tumor budding; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Okuyama K., Fukushima H., Naruse T., Yanamoto S., Tsuchihashi H., Umeda M. CD44 Variant 6 Expression and Tumor Budding in the Medullary Invasion Front of Mandibular Gingival Squamous Cell Carcinoma Are Predictive Factors for Cervical Lymph Node Metastasis. Pathol. Oncol. Res. 2019;25:603–609. doi: 10.1007/s12253-018-0529-y. - DOI - PubMed
-
- Attramadal C.G., Kumar S., Boysen M.E., Dhakal H.P., Nesland J.M., Bryne M. Tumor Budding, EMT and Cancer Stem Cells in T1-2/N0 Oral Squamous Cell Carcinomas. Anticancer Res. 2015;35:6111–6120. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

