10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis
- PMID: 36831468
- PMCID: PMC9954579
- DOI: 10.3390/cancers15041125
10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis
Abstract
Background: Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel intraperitoneal drug delivery method of low-dose chemotherapy as a pressurized aerosol in patients affected by peritoneal cancer of primary or secondary origin. We performed a systematic review and meta-analysis with the aim of assessing the feasibility, safety, and efficacy of PIPAC.
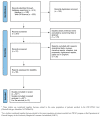
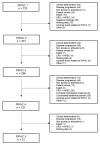
Methods: A systematic literature search was performed using Medline and Web of Science databases from 1 January 2011, to inception, to 31 December 2021. Data were independently extracted by two authors. The Newcastle-Ottawa Scale was used to assess the quality and risk of bias of studies. Meta-analysis was performed for pathological response, radiological response, PCI variation along treatment, and for patients undergoing three or more PIPAC. Pooled analyses were performed using the Freeman-Tukey double arcsine transformation, and 95% CIs were calculated using Clopper-Pearson exact CIs in all instances.
Results: A total of 414 papers on PIPAC were identified, and 53 studies considering 4719 PIPAC procedure in 1990 patients were included for analysis. The non-access rate or inability to perform PIPAC pooled rate was 4% of the procedures performed. The overall proportion of patients who completed 3 or more cycles of PIPAC was 39%. Severe toxicities considering CTCAE 3-4 were 4% (0% to 38.5%). In total, 50 studies evaluated deaths within the first 30 postoperative days. In the included 1936 patients were registered 26 deaths (1.3%). The pooled analysis of all the studies reporting a pathological response was 68% (95% CI 0.61-0.73), with an acceptable heterogeneity (I2 28.41%, p = 0.09). In total, 10 papers reported data regarding the radiological response, with high heterogeneity and a weighted means of 15% (0% to 77.8%). PCI variation along PIPAC cycles were reported in 14 studies. PCI diminished, increased, or remained stable in eight, one and five studies, respectively, with high heterogeneity at pooled analysis. Regarding survival, there was high heterogeneity. The 12-month estimated survival from first PIPAC for colorectal cancer, gastric cancer, gynecological cancer and hepatobiliary/pancreatic cancer were, respectively, 53%, 25%, 59% and 37%.
Conclusions: PIPAC may be a useful treatment option for selected patients with PM, with acceptable grade 3 and 4 toxicity and promising survival benefit. Meta-analysis showed high heterogeneity of data among up-to-date available studies. In a subset analysis per primary tumor origin, pathological tumor regression was documented in 68% of the studies with acceptable heterogeneity. Pathological regression seems, therefore, a reliable outcome for PIPAC activity and a potential surrogate endpoint of treatment response. We recommend uniform selection criteria for patients entering a PIPAC program and highlight the urgent need to standardize items for PIPAC reports and datasets.
Keywords: aerosol chemotherapy; carcinomatosis; locoregional chemotherapy; neoadjuvant treatment; peritoneal metastases; pressurized intraperitoneal aerosol chemotherapy (PIPAC); response assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Higgins J., James T., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) [(accessed on 5 November 2022)]. Available online: https://training.cochrane.org/handbook/current.
-
- PROSPERO. [(accessed on 5 November 2022)]. Available online: https://www.crd.york.ac.uk/prospero/
-
- Ferracci F., Di Giorgio A., Robella M., Macrì A. 10 Years of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastases: A Systematic Review and Meta- Analysis. PROSPERO 2022 CRD42022320389. PROSPERO Int. Prospect. Regist. Syst. Rev. 2022:1–4.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

