Neurofibromatosis Type 1: Pediatric Aspects and Review of Genotype-Phenotype Correlations
- PMID: 36831560
- PMCID: PMC9954221
- DOI: 10.3390/cancers15041217
Neurofibromatosis Type 1: Pediatric Aspects and Review of Genotype-Phenotype Correlations
Abstract
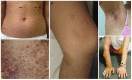
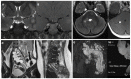
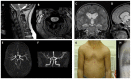
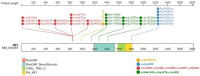
Neurofibromatosis type 1 (NF1) is an autosomal dominant condition, with a birth incidence of approximately 1:2000-3000, caused by germline pathogenic variants in NF1, a tumor suppressor gene encoding neurofibromin, a negative regulator of the RAS/MAPK pathway. This explains why NF1 is included in the group of RASopathies and shares several clinical features with Noonan syndrome. Here, we describe the main clinical characteristics and complications associated with NF1, particularly those occurring in pediatric age. NF1 has complete penetrance and shows wide inter- and intrafamilial phenotypic variability and age-dependent appearance of manifestations. Clinical presentation and history of NF1 are multisystemic and highly unpredictable, especially in the first years of life when penetrance is still incomplete. In this scenario of extreme phenotypic variability, some genotype-phenotype associations need to be taken into consideration, as they strongly impact on genetic counseling and prognostication of the disease. We provide a synthetic review, based on the most recent literature data, of all known genotype-phenotype correlations from a genetic and clinical perspective. Molecular diagnosis is fundamental for the confirmation of doubtful clinical diagnoses, especially in the light of recently revised diagnostic criteria, and for the early identification of genotypes, albeit few, that correlate with specific phenotypes.
Keywords: genotype–phenotype correlations; neurofibromatosis type 1 (NF1); pediatric features.
Conflict of interest statement
C.S.: paid consulting or advisory role for AstraZeneca. The other authors declare no conflict of interest.
Figures


References
-
- Friedman J.M. Neurofibromatosis 1. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

