MiR-21 Is Induced by Hypoxia and Down-Regulates RHOB in Prostate Cancer
- PMID: 36831632
- PMCID: PMC9954526
- DOI: 10.3390/cancers15041291
MiR-21 Is Induced by Hypoxia and Down-Regulates RHOB in Prostate Cancer
Abstract
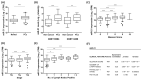
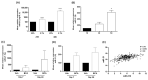
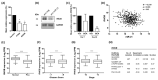
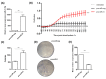
Tumour hypoxia is a well-established contributor to prostate cancer progression and is also known to alter the expression of several microRNAs. The over-expression of microRNA-21 (miR-21) has been consistently linked with many cancers, but its role in the hypoxic prostate tumour environment has not been well studied. In this paper, the link between hypoxia and miR-21 in prostate cancer is investigated. A bioinformatic analysis of The Cancer Genome Atlas (TCGA) prostate biopsy datasets shows the up-regulation of miR-21 is significantly associated with prostate cancer and clinical markers of disease progression. This up-regulation of miR-21 expression was shown to be caused by hypoxia in the LNCaP prostate cancer cell line in vitro and in an in vivo prostate tumour xenograft model. A functional enrichment analysis also revealed a significant association of miR-21 and its target genes with processes related to cellular hypoxia. The over-expression of miR-21 increased the migration and colony-forming ability of RWPE-1 normal prostate cells. In vitro and in silico analyses demonstrated that miR-21 down-regulates the tumour suppressor gene Ras Homolog Family Member B (RHOB) in prostate cancer. Further a TCGA analysis illustrated that miR-21 can distinguish between different patient outcomes following therapy. This study presents evidence that hypoxia is a key contributor to the over-expression of miR-21 in prostate tumours, which can subsequently promote prostate cancer progression by suppressing RHOB expression. We propose that miR-21 has good potential as a clinically useful diagnostic and prognostic biomarker of hypoxia and prostate cancer.
Keywords: RHOB; biomarker; hypoxia; miR-21; microRNA; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McKenna D.J., Errington R., Pors K. Current challenges and opportunities in treating hypoxic prostate tumors. J. Cancer Metastasis Treat. 2018;4:11. doi: 10.20517/2394-4722.2017.54. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

