Downstream Targets of VHL/HIF-α Signaling in Renal Clear Cell Carcinoma Progression: Mechanisms and Therapeutic Relevance
- PMID: 36831657
- PMCID: PMC9953937
- DOI: 10.3390/cancers15041316
Downstream Targets of VHL/HIF-α Signaling in Renal Clear Cell Carcinoma Progression: Mechanisms and Therapeutic Relevance
Abstract
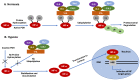
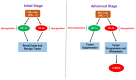
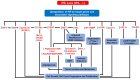
The clear cell variant of renal cell carcinoma (ccRCC) is the most common renal epithelial malignancy and responsible for most of the deaths from kidney cancer. Patients carrying inactivating mutations in the Von Hippel-Lindau (VHL) gene have an increased proclivity to develop several types of tumors including ccRCC. Normally, the Hypoxia Inducible Factor alpha (HIF-α) subunits of the HIF heterodimeric transcription factor complex are regulated by oxygen-dependent prolyl-hydroxylation, VHL-mediated ubiquitination and proteasomal degradation. Loss of pVHL function results in elevated levels of HIF-α due to increased stability, leading to RCC progression. While HIF-1α acts as a tumor suppressor, HIF-2α promotes oncogenic potential by driving tumor progression and metastasis through activation of hypoxia-sensitive signaling pathways and overexpression of HIF-2α target genes. One strategy to suppress ccRCC aggressiveness is directed at inhibition of HIF-2α and the associated molecular pathways leading to cell proliferation, angiogenesis, and metastasis. Indeed, clinical and pre-clinical data demonstrated the effectiveness of HIF-2α targeted therapy in attenuating ccRCC progression. This review focuses on the signaling pathways and the involved genes (cyclin D, c-Myc, VEGF-a, EGFR, TGF-α, GLUT-1) that confer oncogenic potential downstream of the VHL-HIF-2α signaling axis in ccRCC. Discussed as well are current treatment options (including receptor tyrosine kinase inhibitors such as sunitinib), the medical challenges (high prevalence of metastasis at the time of diagnosis, refractory nature of advanced disease to current treatment options), scientific challenges and future directions.
Keywords: EGFR; HIF-α; MET; VHL; ccRCC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

