Nano-Electrochemical Characterization of a 3D Bioprinted Cervical Tumor Model
- PMID: 36831668
- PMCID: PMC9954750
- DOI: 10.3390/cancers15041327
Nano-Electrochemical Characterization of a 3D Bioprinted Cervical Tumor Model
Abstract
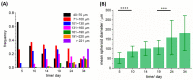
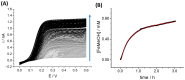
Current cancer research is limited by the availability of reliable in vivo and in vitro models that are able to reproduce the fundamental hallmarks of cancer. Animal experimentation is of paramount importance in the progress of research, but it is becoming more evident that it has several limitations due to the numerous differences between animal tissues and real, in vivo human tissues. 3D bioprinting techniques have become an attractive tool for many basic and applied research fields. Concerning cancer, this technology has enabled the development of three-dimensional in vitro tumor models that recreate the characteristics of real tissues and look extremely promising for studying cancer cell biology. As 3D bioprinting is a relatively recently developed technique, there is still a lack of characterization of the chemical cellular microenvironment of 3D bioprinted constructs. In this work, we fabricated a cervical tumor model obtained by 3D bioprinting of HeLa cells in an alginate-based matrix. Characterization of the spheroid population obtained as a function of culturing time was performed by phase-contrast and confocal fluorescence microscopies. Scanning electrochemical microscopy and platinum nanoelectrodes were employed to characterize oxygen concentrations-a fundamental characteristic of the cellular microenvironment-with a high spatial resolution within the 3D bioprinted cervical tumor model; we also demonstrated that the diffusion of a molecular model of drugs in the 3D bioprinted construct, in which the spheroids were embedded, could be measured quantitatively over time using scanning electrochemical microscopy.
Keywords: 3D bioprinting; cellular microenvironment; cervical tumor model; hypoxia; scanning electrochemical microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

