The Role of Beta-Endorphin in Food Deprivation-Mediated Increases in Food Intake and Binge-Eating
- PMID: 36831755
- PMCID: PMC9954518
- DOI: 10.3390/brainsci13020212
The Role of Beta-Endorphin in Food Deprivation-Mediated Increases in Food Intake and Binge-Eating
Abstract
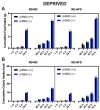
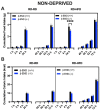
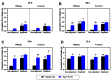
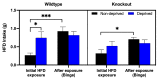
Food deprivation and binge eating represent significant public health concerns. Previous studies have implicated that hypothalamic opioids are affected following food deprivation. However, the role of each opioid peptide is not fully understood. Therefore, we investigated the role of endogenous beta-endorphin in food deprivation-mediated increases in food intake and binge eating. Male mice lacking beta-endorphin and their respective controls were subjected to 24 h food deprivation and then were randomly assigned to receive a regular diet (RD) or a high-fat diet (HFD). After four to five weeks, animals were re-exposed to an HFD to assess if previous exposure to HFD would enhance binge-eating behavior. We report that food deprivation significantly increases food intake; however, beta-endorphin may not be involved in this process. In addition, our findings suggest that prior exposure to an HFD promotes binge-eating behavior in wildtype mice, and that these effects were modestly decreased in beta-endorphin knockout mice. Overall, our results support that beta-endorphin may play a modest role in mediating palatability-driven feeding, but not hunger-associated feeding. A better understanding of neural mechanisms involved in binge eating and deprivation-induced increases in food intake may inspire new prevention or treatment options to decrease the burden of eating disorders.
Keywords: beta-endorphin; binge-eating; food deprivation; high-fat diet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Robinson B., Moran T. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1030–R1036. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

