Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia
- PMID: 36831796
- PMCID: PMC9954117
- DOI: 10.3390/brainsci13020253
Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia
Abstract
Transcutaneous auricular vagus nerve stimulation was recently reported to have a therapeutic potential for functional dyspepsia (FD). This study aimed to explore the integrative effects and mechanisms of auricular vagus nerve stimulation (aVNS) in a rodent model of FD.
Methods: We evaluated the effects of aVNS on visceral hypersensitivity, gastric motility and open field test (OFT) activity in iodoacetamide (IA)-treated rats. The autonomic function was assessed; blood samples and tissues were collected and analyzed by an enzyme-linked immunosorbent assay and western blot. Vagotomy was performed to investigate the role of vagal efferent nerve.
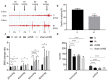
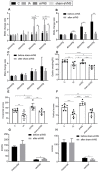
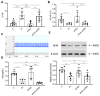
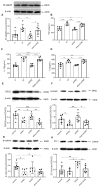
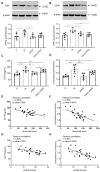
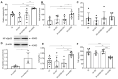
Results: aVNS reduced the electromyography response to gastric distension, improved gastric emptying and increased the horizontal and vertical motion scores of the OFT in IA-treated rats. The sympathovagal ratio was increased in IA-treated rats but normalized with aVNS. The serum cytokines TNF-α, IL-6, IL-1β and NF-κBp65 were increased in IA-treated rats and decreased with aVNS. The hypothalamus-pituitary-adrenal axis was hyperactive in IA-treated rats but inhibited by aVNS. The expression of duodenal desmoglein 2 and occludin were all decreased in IA-treated rats and increased with aVNS but not sham-aVNS. Vagotomy abolished the ameliorating effects of aVNS on gastric emptying, horizontal motions, serum TNF-α and duodenal NF-κBp65.
Conclusion: aVNS improves gastric motility and gastric hypersensitivity probably by anti-inflammatory mechanisms via the vago-vagal pathways. A better understanding on the mechanisms of action involved with aVNS would lead to the optimization of the taVNS methodology and promote taVNS as a non-pharmacological alternative therapy for FD.
Keywords: cholinergic anti-inflammatory pathway; hypothalamic–pituitary-adrenal axis; inflammation; vagotomy; vagus nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Aziz I., Palsson O.S., Tornblom H., Sperber A.D., Whitehead W.E., Simren M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 2018;3:252–262. doi: 10.1016/S2468-1253(18)30003-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

