Apparent Lack of Benefit of Combining Repetitive Transcranial Magnetic Stimulation with Internet-Delivered Cognitive Behavior Therapy for the Treatment of Resistant Depression: Patient-Centered Randomized Controlled Pilot Trial
- PMID: 36831836
- PMCID: PMC9954722
- DOI: 10.3390/brainsci13020293
Apparent Lack of Benefit of Combining Repetitive Transcranial Magnetic Stimulation with Internet-Delivered Cognitive Behavior Therapy for the Treatment of Resistant Depression: Patient-Centered Randomized Controlled Pilot Trial
Abstract
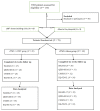
Background: Treatment-resistant depression (TRD) is considered one of the major clinical challenges in the field of psychiatry. An estimated 44% of patients with major depressive disorder (MDD) do not respond to two consecutive antidepressant therapies, and 33% do not respond to up to four antidepressants. Over 15% of all patients with MDD remain refractory to any treatment intervention. rTMS is considered a treatment option for patients with TRD. Likewise, iCBT is evidence-based, symptom-focused psychotherapy recommended for the treatment of TRD. Objective: This study aimed to evaluate the initial comparative clinical effectiveness of rTMS treatment with and without iCBT as an innovative intervention for the treatment of participants diagnosed with TRD. Methods: This study is a prospective two-arm randomized controlled trial. Overall, 78 participants diagnosed with TRD were randomized to one of two treatment interventions: rTMS sessions alone and rTMS sessions plus iCBT. Participants in each group were made to complete evaluation measures at baseline, and 6 weeks (discharge) from treatment. The primary outcome measure was baseline to six weeks change in mean score for the 17-item Hamilton depression rating scale (HAMD-17). Secondary outcomes included mean baseline to six-week changes in the Columbia suicide severity rating scale (CSSRS) for the rate of suicidal ideations, the QIDS-SR16 for subjective depression, and the EQ-5D-5L to assess the quality of health in participants. Results: A majority of the participants were females 50 (64.1%), aged ≥ 40 39 (50.0%), and had college/university education 54 (73.0%). After adjusting for the baseline scores, the study failed to find a significant difference in the changes in mean scores for participants from baseline to six weeks between the two interventions under study on the HAMD-17 scale: F (1, 53) = 0.15, p = 0.70, partial eta squared = 0.003, CSSRS; F (1, 56) = 0.04 p = 0.85, partial eta squared = 0.001, QIDS-SR16 scale; F (1, 53) = 0.04 p = 0.61, partial eta squared = 0.005, and EQ-5D-VAS; F (1, 51) = 0.46 p = 0.50, and partial eta squared = 0.009. However, there was a significant reduction in means scores at week six compared to baseline scores for the combined study population on the HAMD-17 scale (42%), CSSRS (41%), QIDS-SR16 scale (35%), and EQ-VAS scale (62%). Conclusion: This study did not find that combined treatment of TRD with rTMS + iCBT (unguided) was superior to treatment with rTMS alone. Our findings do not support the use of combined treatment of rTMS + iCBT for the management of TRD disorders.
Keywords: MoodGYM; internet-based cognitive behavioral therapy; major depressive disorder; repetitive transcranial magnetic stimulation; treatment-resistant depression.
Conflict of interest statement
The authors declare no conflict of interest.
References
-
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR* D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. - DOI - PubMed
-
- Bourin M. Treatment Resistance in Psychiatry. Springer; Berlin/Heidelberg, Germany: 2019. Developing Therapies for Treatment-Resistant Depressive Disorder in Animal Models; pp. 79–86.
-
- De Berardis D., Tomasetti C., Pompili M., Serafini G., Vellante F., Fornaro M., Valchera A., Perna G., Volpe U., Martinotti G., et al. An Update on Glutamatergic System in Suicidal Depression and on the Role of Esketamine. Curr. Top. Med. Chem. 2020;20:554–584. doi: 10.2174/1568026620666200131100316. - DOI - PubMed
-
- Sackeim H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry. 2001;62:10–17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical