A Portable Wireless Intelligent Nanosensor for 6,7-Dihydroxycoumarin Analysis with A Black Phosphorene and Nano-Diamond Nanocomposite-Modified Electrode
- PMID: 36831920
- PMCID: PMC9953709
- DOI: 10.3390/bios13020153
A Portable Wireless Intelligent Nanosensor for 6,7-Dihydroxycoumarin Analysis with A Black Phosphorene and Nano-Diamond Nanocomposite-Modified Electrode
Abstract
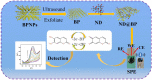
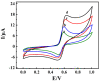
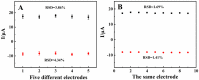
In this work, a novel portable and wireless intelligent electrochemical nanosensor was developed for the detection of 6,7-dihydroxycoumarin (6,7-DHC) using a modified screen-printed electrode (SPE). Black phosphorene (BP) nanosheets were prepared via exfoliation of black phosphorus nanoplates. The BP nanosheets were then mixed with nano-diamond (ND) to prepare ND@BP nanocomposites using the self-assembly method, achieving high environmental stability. The nanocomposite was characterized by SEM, TEM, Raman, XPS and XRD. The nanocomposite was used for the modification of SPE to improve its electrochemical performances. The nanosensor displayed a wide linear range of 0.01-450.0 μmol/L with a low detection limit of 0.003 μmol/L for 6,7-DHC analysis. The portable and wireless intelligent electrochemical nanosensor was applied to detect 6,7-DHC in real drug samples by the standard addition method with satisfactory recoveries, which extends the application of BP-based nanocomposite for electroanalysis.
Keywords: 6,7-Dihydroxycoumarin; black phosphorene nanosheets; electrochemistry; nano-diamond; portable wireless intelligent electrochemical nanosensor; screen-printed electrode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Madureira J., Botelho M.L., Cooper W.J., Leal J.P., Melo R. Aqueous degradation of esculetin (6,7-dihydroxycoumarin) using gamma radiation. Desalin. Water Treat. 2020;181:385–390. doi: 10.5004/dwt.2020.25110. - DOI
-
- Zhao X.J., Zhang F.Y., Lu D.B., Du Y.L., Ye W.C., Wang C.M. Fabrication of a CuS/graphene nanocomposite modified electrode and its application for electrochemical determination of esculetin. Anal. Methods. 2013;5:3992–3998. doi: 10.1039/c3ay40558c. - DOI
-
- Li Y.Y., Song Y.Y., Liu C.H., Huang X.T., Zheng X., Li N., Xu M.L., Mi S.Q., Wang N.S. Simultaneous determination of esculin and its metabolite esculetin in rat plasma by LC–ESI-MS/MS and its application in pharmacokinetic study. J. Chromatogr. B. 2012;907:27–33. doi: 10.1016/j.jchromb.2012.08.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 21964007/The National Natural Science Foundation of China
- YSPTZX202126/The Specific Research Fund of the Innovation Platform for Academicians of Hainan Province
- ZDYF2020204/The Innovation Platform for Academicians of Hainan Province, the Key Research and Development Program of Hainan Province-Social Development Direction
- 2022LTOM01/The Open Foundation of Key Laboratory of Laser Technology and Optoelectronic Functional Materials of Hainan Province
- Hgb 202204/The Graduate Student Innovation Project of the College of Chemistry and Chemical Engineering Hainan Normal University
LinkOut - more resources
Full Text Sources

