Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends
- PMID: 36831978
- PMCID: PMC9953752
- DOI: 10.3390/bios13020211
Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends
Abstract
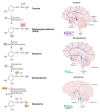
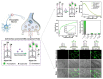
Catecholamines, including dopamine, epinephrine, and norepinephrine, are considered one of the most crucial subgroups of neurotransmitters in the central nervous system (CNS), in which they act at the brain's highest levels of mental function and play key roles in neurological disorders. Accordingly, the analysis of such catecholamines in biological samples has shown a great interest in clinical and pharmaceutical importance toward the early diagnosis of neurological diseases such as Epilepsy, Parkinson, and Alzheimer diseases. As promising routes for the real-time monitoring of catecholamine neurotransmitters, optical and electrochemical biosensors have been widely adopted and perceived as a dramatically accelerating development in the last decade. Therefore, this review aims to provide a comprehensive overview on the recent advances and main challenges in catecholamines biosensors. Particular emphasis is given to electrochemical biosensors, reviewing their sensing mechanism and the unique characteristics brought by the emergence of nanotechnology. Based on specific biosensors' performance metrics, multiple perspectives on the therapeutic use of nanomaterial for catecholamines analysis and future development trends are also summarized.
Keywords: catecholamine neurotransmitters; dopamine; electrochemical biosensor; epinephrine; nanomaterials; norepinephrine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Lončar A., Negrojević L., Dimitrić-Marković J., Dimić D. The Reactivity of Neurotransmitters and Their Metabolites towards Various Nitrogen-Centered Radicals: Experimental, Theoretical, and Biotoxicity Evaluation. Comput. Biol. Chem. 2021;95:107573. doi: 10.1016/j.compbiolchem.2021.107573. - DOI - PubMed
-
- Roondhe B., Jha P.K. Neurotransmitter-Functionalized Boron Nitride Nanoribbons as Biological Cargo Carriers: Analysis by Density Functional Theory. ACS Appl. Nano Mater. 2019;2:1552–1561. doi: 10.1021/acsanm.9b00028. - DOI
-
- Xie W., Yin Y., Gu R., Xu J., Su X., Wang Y., Liu R., Liu X., Huang J. Label-Free and Highly Selective MOFs-Based Dopamine Detection in Urine of Parkinson’s Patients. Chem. Eng. J. 2022;443:136371. doi: 10.1016/j.cej.2022.136371. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

