Diboronic-Acid-Based Electrochemical Sensor for Enzyme-Free Selective and Sensitive Glucose Detection
- PMID: 36832014
- PMCID: PMC9954471
- DOI: 10.3390/bios13020248
Diboronic-Acid-Based Electrochemical Sensor for Enzyme-Free Selective and Sensitive Glucose Detection
Abstract
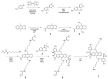
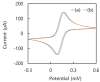
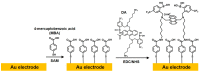
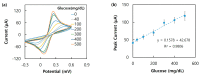
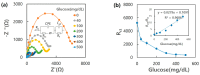
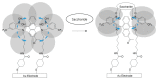
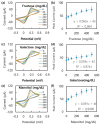
A diboronic acid anthracene-based fluorescent system for detecting blood glucose could be used for 180 days. However, there has not yet been a boronic acid immobilized electrode to selectively detect glucose in a signal-increased way. Considering malfunctions of sensors at high sugar levels, the electrochemical signal should be increased proportionally to the glucose concentration. Therefore, we synthesized a new diboronic acid derivative and fabricated the derivative-immobilized electrodes for the selective detection of glucose. We performed cyclic voltammetry and electrochemical impedance spectroscopy with an Fe(CN)63-/4- redox pair for detecting glucose in the range of 0-500 mg/dL. The analysis revealed increased electron-transfer kinetics such as increased peak current and decreased semicircle radius of Nyquist plots as the glucose concentration increased. The cyclic voltammetry and impedance spectroscopy showed that the linear detection range of glucose was 40 to 500 mg/dL with limits of detection of 31.2 mg/dL and 21.5 mg/dL, respectively. We applied the fabricated electrode to detect glucose in artificial sweat and obtained 90% of the performance of the electrodes in PBS. Cyclic voltammetry measurements of other sugars such as galactose, fructose, and mannitol also showed linear increased peak currents proportional to the concentrations of the tested sugars. However, the slopes of the sugars were lower than that of glucose, indicating selectivity for glucose. These results proved the newly synthesized diboronic acid is a promising synthetic receptor for developing a long-term usable electrochemical sensor system.
Keywords: boronic acid; diabetes; electrochemical sensor; glucose monitoring; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- IDF Diabetes Atlas, 10th ed. 2021. [(accessed on 21 November 2022)]. Available online: https://diabetesatlas.org/
-
- Bandodkar A., Jia W., Yardımcı C., Wang X., Ramirez J., Wang J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2014;87:394. - PubMed
-
- Martín A., Kim J., Kurniawan J., Sempionatto J., Moreto J., Tang G., Campbell A., Shin A., Lee M.Y., Liu X., et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017;2:1860. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

