Recent Advances on Peptide-Based Biosensors and Electronic Noses for Foodborne Pathogen Detection
- PMID: 36832024
- PMCID: PMC9954637
- DOI: 10.3390/bios13020258
Recent Advances on Peptide-Based Biosensors and Electronic Noses for Foodborne Pathogen Detection
Abstract
Foodborne pathogens present a serious issue around the world due to the remarkably high number of illnesses they cause every year. In an effort to narrow the gap between monitoring needs and currently implemented classical detection methodologies, the last decades have seen an increased development of highly accurate and reliable biosensors. Peptides as recognition biomolecules have been explored to develop biosensors that combine simple sample preparation and enhanced detection of bacterial pathogens in food. This review first focuses on the selection strategies for the design and screening of sensitive peptide bioreceptors, such as the isolation of natural antimicrobial peptides (AMPs) from living organisms, the screening of peptides by phage display and the use of in silico tools. Subsequently, an overview on the state-of-the-art techniques in the development of peptide-based biosensors for foodborne pathogen detection based on various transduction systems was given. Additionally, limitations in classical detection strategies have led to the development of innovative approaches for food monitoring, such as electronic noses, as promising alternatives. The use of peptide receptors in electronic noses is a growing field and the recent advances of such systems for foodborne pathogen detection are presented. All these biosensors and electronic noses are promising alternatives for the pathogen detection with high sensitivity, low cost and rapid response, and some of them are potential portable devices for on-site analyses.
Keywords: antimicrobial peptides; biosensors; electronic nose; foodborne pathogen; pathogenic detection; peptides; phage display.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
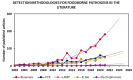
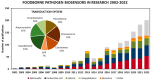












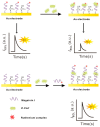



References
-
- World Health Organization (WHO) Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; Geneva, Switzerland: 2015.
-
- Desta Sisay M.A. A Review on Major Food Borne Bacterial Illnesses. J. Trop. Dis. 2015;3:176. doi: 10.4172/2329-891X.1000176. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

