Microfluidic Wound-Healing Assay for ECM and Microenvironment Properties on Microglia BV2 Cells Migration
- PMID: 36832056
- PMCID: PMC9954450
- DOI: 10.3390/bios13020290
Microfluidic Wound-Healing Assay for ECM and Microenvironment Properties on Microglia BV2 Cells Migration
Abstract
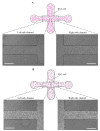
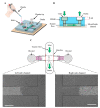
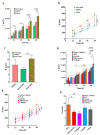
Microglia cells, as the resident immune cells of the central nervous system (CNS), are highly motile and migratory in development and pathophysiological conditions. During their migration, microglia cells interact with their surroundings based on the various physical and chemical properties in the brain. Herein, a microfluidic wound-healing chip is developed to investigate microglial BV2 cell migration on the substrates coated with extracellular matrixes (ECMs) and substrates usually used for bio-applications on cell migration. In order to generate the cell-free space (wound), gravity was utilized as a driving force to flow the trypsin with the device. It was shown that, despite the scratch assay, the cell-free area was created without removing the extracellular matrix coating (fibronectin) using the microfluidic assay. It was found that the substrates coated with Poly-L-Lysine (PLL) and gelatin stimulated microglial BV2 migration, while collagen and fibronectin coatings had an inhibitory effect compared to the control conditions (uncoated glass substrate). In addition, the results showed that the polystyrene substrate induced higher cell migration than the PDMS and glass substrates. The microfluidic migration assay provides an in vitro microenvironment closer to in vivo conditions for further understanding the microglia migration mechanism in the brain, where the environment properties change under homeostatic and pathological conditions.
Keywords: cell migration; extracellular matrix coating; microfluidic wound-healing migration assay; microglia cells; substrate rigidity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Ortega-Carrion A., Feo-Lucas L., Vicente-Manzanares M. Cell Migration. Encycl. Cell Biol. 2016;2:720–730. doi: 10.1016/b978-0-12-394447-4.20070-9. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

