Non-Invasive Retinal Imaging Modalities for the Identification of Prognostic Factors in Vitreoretinal Surgery for Full-Thickness Macular Holes
- PMID: 36832078
- PMCID: PMC9955111
- DOI: 10.3390/diagnostics13040589
Non-Invasive Retinal Imaging Modalities for the Identification of Prognostic Factors in Vitreoretinal Surgery for Full-Thickness Macular Holes
Abstract
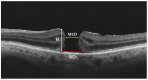
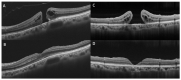
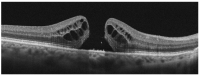
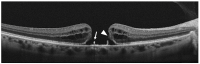
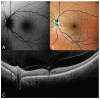
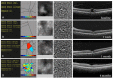
In this review, we will focus on different non-invasive retinal imaging techniques that can be used to evaluate morphological and functional features in full-thickness macular holes with a prognostic purpose. Technological innovations and developments in recent years have increased the knowledge of vitreoretinal interface pathologies by identifying potential biomarkers useful for surgical outcomes prediction. Despite a successful surgery of full-thickness macular holes, the visual outcomes are often puzzling, so the study and the identification of prognostic factors is a current topic of interest. Our review aims to provide an overview of the current knowledge on prognostic biomarkers identified in full-thickness macular holes by means of different retinal imaging tools, such as optical coherence tomography, optical coherence tomography angiography, microperimetry, fundus autofluorescence, and adaptive optics.
Keywords: OCT; OCTA; biomarkers; full-thickness macular hole; prognostic factors; retinal imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Morpho-Functional Evaluation of Full-Thickness Macular Holes by the Integration of Optical Coherence Tomography Angiography and Microperimetry.J Clin Med. 2020 Jan 15;9(1):229. doi: 10.3390/jcm9010229. J Clin Med. 2020. PMID: 31952306 Free PMC article.
-
Optical coherence tomography angiography features in patients with idiopathic full-thickness macular hole, before and after surgical treatment.Clin Interv Aging. 2019 Mar 8;14:505-514. doi: 10.2147/CIA.S189417. eCollection 2019. Clin Interv Aging. 2019. PMID: 30880931 Free PMC article.
-
Optical coherence tomography of macular holes.Ophthalmology. 1995 May;102(5):748-56. doi: 10.1016/s0161-6420(95)30959-1. Ophthalmology. 1995. PMID: 7777274
-
Microstructural and hemodynamic changes in the fundus after pars plana vitrectomy for different vitreoretinal diseases.Graefes Arch Clin Exp Ophthalmol. 2024 Jul;262(7):1977-1992. doi: 10.1007/s00417-023-06303-x. Epub 2023 Nov 20. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 37982887 Review.
-
[Biomarkers in full-thickness and lamellar defects of the macula].Ophthalmologe. 2021 Apr;118(4):321-336. doi: 10.1007/s00347-021-01340-5. Epub 2021 Mar 1. Ophthalmologe. 2021. PMID: 33646383 Review. German.
Cited by
-
Prediction of long-term visual outcome of idiopathic full-thickness macular hole surgery using optical coherence tomography parameters that estimate potential preoperative photoreceptor damage.Graefes Arch Clin Exp Ophthalmol. 2024 Oct;262(10):3181-3189. doi: 10.1007/s00417-024-06500-2. Epub 2024 May 8. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 38717606 Free PMC article.
-
Surgeon's perceptions and preferences in the management of idiopathic macular hole.Indian J Ophthalmol. 2025 Jan 1;73(Suppl 1):S83-S87. doi: 10.4103/IJO.IJO_1617_24. Epub 2024 Dec 24. Indian J Ophthalmol. 2025. PMID: 39723870 Free PMC article.
References
-
- Meuer S.M., Myers C.E., Klein B.E., Swift M.K., Huang Y., Gangaputra S., Pak J.W., Danis R.P., Klein R. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The beaver dam eye study. Ophthalmology. 2015;122:787–795. doi: 10.1016/j.ophtha.2014.10.014. - DOI - PMC - PubMed
-
- Duker J.S., Kaiser P.K., Binder S., de Smet M.D., Gaudric A., Reichel E., Sadda S.R., Sebag J., Spaide R.F., Stalmans P. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–2619. doi: 10.1016/j.ophtha.2013.07.042. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

