Low-Dose CT Fluoroscopy-Guided Drainage of Deep Pelvic Fluid Collections after Colorectal Cancer Surgery: Technical Success, Clinical Outcome and Safety in 40 Patients
- PMID: 36832199
- PMCID: PMC9955776
- DOI: 10.3390/diagnostics13040711
Low-Dose CT Fluoroscopy-Guided Drainage of Deep Pelvic Fluid Collections after Colorectal Cancer Surgery: Technical Success, Clinical Outcome and Safety in 40 Patients
Abstract
Purpose: To assess the technical (TS) and clinical success (CS) of CT fluoroscopy-guided drainage (CTD) in patients with symptomatic deep pelvic fluid collections following colorectal surgery.
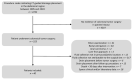
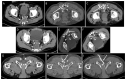
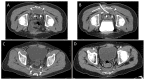
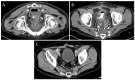
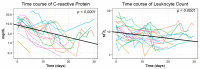
Methods: A retrospective analysis (years 2005 to 2020) comprised 43 drain placements in 40 patients undergoing low-dose (10-20 mA tube current) quick-check CTD using a percutaneous transgluteal (n = 39) or transperineal (n = 1) access. TS was defined as sufficient drainage of the fluid collection by ≥50% and the absence of complications according to the Cardiovascular and Interventional Radiological Society of Europe (CIRSE). CS comprised the marked reduction of elevated laboratory inflammation parameters by ≥50% under minimally invasive combination therapy (i.v. broad-spectrum antibiotics, drainage) within 30 days after intervention and no surgical revision related to the intervention required.
Results: TS was gained in 93.0%. CS was obtained in 83.3% for C-reactive Protein and in 78.6% for Leukocytes. In five patients (12.5%), a reoperation due to an unfavorable clinical outcome was necessary. Total dose length product (DLP) tended to be lower in the second half of the observation period (median: years 2013 to 2020: 544.0 mGy*cm vs. years 2005 to 2012: 735.5 mGy*cm) and was significantly lower for the CT fluoroscopy part (median: years 2013 to 2020: 47.0 mGy*cm vs. years 2005 to 2012: 85.0 mGy*cm).
Conclusions: Given a minor proportion of patients requiring surgical revision due to anastomotic leakage, the CTD of deep pelvic fluid collections is safe and provides an excellent technical and clinical outcome. The reduction of radiation exposition over time can be achieved by both the ongoing development of CT technology and the increased level of interventional radiology (IR) expertise.
Keywords: CT-guided drainage; clinical outcome; colorectal surgery; pelvic fluid collection; technical outcome.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Technical and Clinical Outcome of Low-Milliampere CT Fluoroscopy-Guided Percutaneous Drainage Placement in Abdominal Fluid Collections after Liver Transplantation: A 16-Year Retrospective Analysis of 50 Consecutive Patients.Diagnostics (Basel). 2024 Feb 6;14(4):353. doi: 10.3390/diagnostics14040353. Diagnostics (Basel). 2024. PMID: 38396392 Free PMC article.
-
Low-Milliampere CT Fluoroscopy-Guided Percutaneous Drainage Placement after Pancreatic Surgery: Technical and Clinical Outcome in 133 Consecutive Patients during a 14-Year Period.Diagnostics (Basel). 2022 Sep 16;12(9):2243. doi: 10.3390/diagnostics12092243. Diagnostics (Basel). 2022. PMID: 36140644 Free PMC article.
-
Intermittent quick-check CT fluoroscopy-guided percutaneous drainage placement in patients with infected renal and perirenal fluid collections: 11-year experience.Diagn Interv Radiol. 2021 May;27(3):378-385. doi: 10.5152/dir.2021.20068. Diagn Interv Radiol. 2021. PMID: 34003125 Free PMC article.
-
Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review.AJR Am J Roentgenol. 2011 Jul;197(1):241-6. doi: 10.2214/AJR.10.5447. AJR Am J Roentgenol. 2011. PMID: 21701036 Review.
-
Percutaneous, computed tomography-guided drainage of deep pelvic abscesses via a transgluteal approach: a report on 30 cases and a review of the literature.Abdom Imaging. 2013 Apr;38(2):285-9. doi: 10.1007/s00261-012-9917-z. Abdom Imaging. 2013. PMID: 22684488 Review.
Cited by
-
Technical and Clinical Outcome of Low-Milliampere CT Fluoroscopy-Guided Percutaneous Drainage Placement in Abdominal Fluid Collections after Liver Transplantation: A 16-Year Retrospective Analysis of 50 Consecutive Patients.Diagnostics (Basel). 2024 Feb 6;14(4):353. doi: 10.3390/diagnostics14040353. Diagnostics (Basel). 2024. PMID: 38396392 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

