Disentangling the Cost of Orphan Drugs Marketed in the United States
- PMID: 36833091
- PMCID: PMC9957503
- DOI: 10.3390/healthcare11040558
Disentangling the Cost of Orphan Drugs Marketed in the United States
Abstract
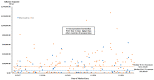
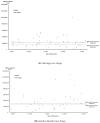
The increasing number and high prices of orphan drugs have triggered concern among patients, payers, and policymakers about the affordability of new drugs approved using the incentives set by the Orphan Drug Act (ODA) of 1983. This study evaluated the factors associated to the differences in the treatment cost of new orphan and non-orphan drugs approved by the FDA from 2017 to 2021. A generalized linear model (GLM) with the Gamma log-link analysis was used to ascertain the association of drug characteristics with the treatment costs of orphan and non-orphan drugs. The results of the study showed that the median and interquartile range (IQR) drug cost was USD 218,872 (IQR = USD 23,105) for orphan drugs and USD 12,798 (IQR = USD 57,940) for non-orphan drugs (p < 0.001). Higher market entry prices were associated with biologics (108%; p < 0.001), orphan status (177%; p < 0.001), US sponsor companies (48%; p = 0.035), chronic use (1083%; p < 0.001), treatment intent (163%; p = 0.004), and indications for oncology (624%; p < 0.001) or genetic disorders (624%; p < 0.001). Higher market entry treatment cost for newly approved drugs were associated with biologics, orphan status, US sponsor companies, chronic use, therapeutic intent, and indications for oncology or genetic disorders.
Keywords: market entry; non-orphan drugs; orphan drugs; price.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Waxman H.A. H.R.5238-97th Congress (1981-1982): Orphan Drug Act. [(accessed on 2 November 2021)];1983 Available online: https://www.congress.gov/bill/97th-congress/house-bill/5238.
-
- FAQs about Rare Diseases|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. [(accessed on 2 November 2021)]; Available online: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-dise....
-
- US Food and Drug Administration Orphan Drug Act-Relevant Excerpts. [(accessed on 5 November 2021)]; Available online: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biolog....
-
- Szydlo R. Office of Orphan Products Development: Financial Incentives for CDER Medical Products. [(accessed on 11 March 2021)]; Available online: https://www.fda.gov/media/135236/download.
-
- Aitken M., Kleinrock M., Muñoz E., Porwal U. Orphan Drugs in the United States: Rare Disease Innovation and Cost Trends Through 2019. IQVIA. [(accessed on 5 November 2021)]. Available online: https://rarediseases.org/wp-content/uploads/2021/03/orphan-drugs-in-theu....
LinkOut - more resources
Full Text Sources