Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability-A Potential Occult Male Factor
- PMID: 36833166
- PMCID: PMC9957300
- DOI: 10.3390/genes14020239
Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability-A Potential Occult Male Factor
Abstract
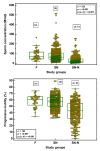
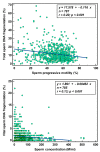
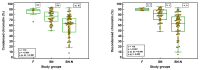
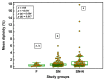
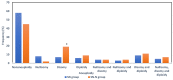
Semen parameters are unable to inform on the function or fertilizing capacity of the male gamete. Standardized methods are provided by the WHO but, the lower reference limits have reduced sensitivity to predict chances of conception. Subfertile men may be falsely classified as "normal" and a male factor contributing to genome instability may be overlooked. Semen parameters, sperm DNA fragmentation (SDF), sperm chromatin maturity and stability, and sperm aneuploidy were assessed in fertile (F), subfertile normozoospermic (SN) and subfertile non-normozoospermic males (SN-N). Standardized assays employing flow cytometry were used to detect genome instability. Sperm DNA fragmentation did not differ significantly whether the semen samples were from a fertile (F), subfertile normozoospermic (SN) or subfertile non-normozoospermic male (SN-N). Chromatin decondensation was significantly reduced and hyperstability significantly increased in the SN group as compared to the F group. The frequency of diploidy was significantly different in the three study groups with significance between F and SN and between F and SN-N groups. Subfertile men with normal semen parameters are often excluded from extensive genetic testing. Genome instability might be an independent attribute of semen quality detecting problems not seen with semen analysis alone.
Keywords: chromatin maturity; genome instability; male factor; normozoospermia; semen parameters; sperm DNA fragmentation; sperm aneuploidy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed. WHO Press; Geneva, Switzerland: 2021. [(accessed on 29 January 2021)]. Available online: https://www.who.int/publications/i/item/9789240030787.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

