Seasonal Adaptation: Geographic Photoperiod-Temperature Patterns Explain Genetic Variation in the Common Vole Tsh Receptor
- PMID: 36833219
- PMCID: PMC9957289
- DOI: 10.3390/genes14020292
Seasonal Adaptation: Geographic Photoperiod-Temperature Patterns Explain Genetic Variation in the Common Vole Tsh Receptor
Abstract
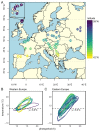
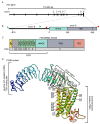
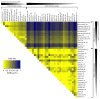
The vertebrate photoperiodic neuroendocrine system uses the photoperiod as a proxy to time the annual rhythms in reproduction. The thyrotropin receptor (TSHR) is a key protein in the mammalian seasonal reproduction pathway. Its abundance and function can tune sensitivity to the photoperiod. To investigate seasonal adaptation in mammals, the hinge region and the first part of the transmembrane domain of the Tshr gene were sequenced for 278 common vole (Microtus arvalis) specimens from 15 localities in Western Europe and 28 localities in Eastern Europe. Forty-nine single nucleotide polymorphisms (SNPs; twenty-two intronic and twenty-seven exonic) were found, with a weak or lack of correlation with pairwise geographical distance, latitude, longitude, and altitude. By applying a temperature threshold to the local photoperiod-temperature ellipsoid, we obtained a predicted critical photoperiod (pCPP) as a proxy for the spring onset of local primary food production (grass). The obtained pCPP explains the distribution of the genetic variation in Tshr in Western Europe through highly significant correlations with five intronic and seven exonic SNPs. The relationship between pCPP and SNPs was lacking in Eastern Europe. Thus, Tshr, which plays a pivotal role in the sensitivity of the mammalian photoperiodic neuroendocrine system, was targeted by natural selection in Western European vole populations, resulting in the optimized timing of seasonal reproduction.
Keywords: Microtus arvalis; Tsh receptor; climate change; common vole; natural selection; seasonal reproduction; temperature–photoperiod ellipsoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Baker J. Evolution, Essays on Aspects of Ev. Biology. Oxford University Press; New York, NY, USA: 1938. The Evolution of Breeding Seasons; pp. 161–177.
-
- Robson M.J. A Comparison of British and North African Varieties of Tall Fescue (Festuca arundinacea). I. Leaf Growth during Winter and the Effects on it of Temperature and Daylength. J. Appl. Ecol. 1967;4:475–484. doi: 10.2307/2401349. - DOI
-
- Peacock J.M. Temperature and Leaf Growth in Four Grass Species. J. Appl. Ecol. 1976;13:225–232. doi: 10.2307/2401942. - DOI
-
- Malyshev A.V., Henry H.A.L., Kreyling J. Relative Effects of Temperature vs Photoperiod on Growth and Cold Acclimation of Northern and Southern Ecotypes of the Grass Arrhenatherum elatius. Environ. Exp. Bot. 2014;106:189–196. doi: 10.1016/j.envexpbot.2014.02.007. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

