Telomere Fragility and MiDAS: Managing the Gaps at the End of the Road
- PMID: 36833275
- PMCID: PMC9956152
- DOI: 10.3390/genes14020348
Telomere Fragility and MiDAS: Managing the Gaps at the End of the Road
Abstract
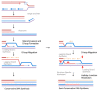
Telomeres present inherent difficulties to the DNA replication machinery due to their repetitive sequence content, formation of non-B DNA secondary structures, and the presence of the nucleo-protein t-loop. Especially in cancer cells, telomeres are hot spots for replication stress, which can result in a visible phenotype in metaphase cells termed "telomere fragility". A mechanism cells employ to mitigate replication stress, including at telomeres, is DNA synthesis in mitosis (MiDAS). While these phenomena are both observed in mitotic cells, the relationship between them is poorly understood; however, a common link is DNA replication stress. In this review, we will summarize what is known to regulate telomere fragility and telomere MiDAS, paying special attention to the proteins which play a role in these telomere phenotypes.
Keywords: DNA replication; Mitotic DNA synthesis; replication stress; telomere fragility; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
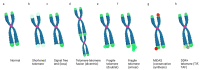

Similar articles
-
Alternative Lengthening of Telomeres Mediated by Mitotic DNA Synthesis Engages Break-Induced Replication Processes.Mol Cell Biol. 2017 Sep 26;37(20):e00226-17. doi: 10.1128/MCB.00226-17. Print 2017 Oct 15. Mol Cell Biol. 2017. PMID: 28760773 Free PMC article.
-
Human cancer cells utilize mitotic DNA synthesis to resist replication stress at telomeres regardless of their telomere maintenance mechanism.Oncotarget. 2018 Mar 23;9(22):15836-15846. doi: 10.18632/oncotarget.24745. eCollection 2018 Mar 23. Oncotarget. 2018. PMID: 29662610 Free PMC article.
-
Resolving Roadblocks to Telomere Replication.Methods Mol Biol. 2019;1999:31-57. doi: 10.1007/978-1-4939-9500-4_2. Methods Mol Biol. 2019. PMID: 31127568 Review.
-
Challenging endings: How telomeres prevent fragility.Bioessays. 2021 Oct;43(10):e2100157. doi: 10.1002/bies.202100157. Epub 2021 Aug 26. Bioessays. 2021. PMID: 34436787 Review.
-
Replication stress conferred by POT1 dysfunction promotes telomere relocalization to the nuclear pore.Genes Dev. 2020 Dec 1;34(23-24):1619-1636. doi: 10.1101/gad.337287.120. Epub 2020 Oct 29. Genes Dev. 2020. PMID: 33122293 Free PMC article.
Cited by
-
Replication stress as a driver of cellular senescence and aging.Commun Biol. 2024 May 22;7(1):616. doi: 10.1038/s42003-024-06263-w. Commun Biol. 2024. PMID: 38777831 Free PMC article. Review.
-
PARP2 promotes Break Induced Replication-mediated telomere fragility in response to replication stress.Nat Commun. 2024 Apr 2;15(1):2857. doi: 10.1038/s41467-024-47222-7. Nat Commun. 2024. PMID: 38565848 Free PMC article.
-
Special Issue "DNA Replication/Repair, and the DNA Damage Response in Human Disease".Genes (Basel). 2023 Apr 11;14(4):893. doi: 10.3390/genes14040893. Genes (Basel). 2023. PMID: 37107651 Free PMC article.
-
The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells.Genes (Basel). 2025 Feb 3;16(2):188. doi: 10.3390/genes16020188. Genes (Basel). 2025. PMID: 40004517 Free PMC article. Review.
-
Oxidative Base Damage to Telomeres Sensitizes Cancer Cells to ATR Inhibition.bioRxiv [Preprint]. 2025 May 13:2025.05.10.653274. doi: 10.1101/2025.05.10.653274. bioRxiv. 2025. PMID: 40463079 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

