Vertical Transmission of Coxsackievirus A6 with Severe Congenital Pneumonia/Sepsis
- PMID: 36833540
- PMCID: PMC9957077
- DOI: 10.3390/ijerph20042843
Vertical Transmission of Coxsackievirus A6 with Severe Congenital Pneumonia/Sepsis
Abstract
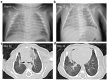
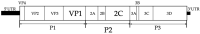
We report a case of vertical transmission of Coxsackievirus (CV)-A6 with severe congenital pneumonia/sepsis. A male infant presented with severe respiratory symptoms at birth and was treated with full cardiopulmonary support, including inhaled nitric oxide. Three days before delivery, his older brother was diagnosed with hand, foot, and mouth disease (HFMD). His mother developed transient fever 1 day before delivery and presented a blister on her thumb 2 days after delivery. A multiplex polymerase chain reaction test on day 2 was positive for human rhinovirus/enterovirus. CV-A6 was later detected in the serum, tracheal aspirate, and stool of the patient sampled on day 6, and in the maternal serum sampled on the day of delivery. He was diagnosed with congenital CV-A6 pneumonia/sepsis caused by vertical transmission, based on VP1 consensus sequences used for typing of the virus that demonstrated a 100% match between the mother and infant. Further, the strain was closely related to the lethal CV-A6-Changchun strains in the phylogenetic analysis of the P2 region, which contributes to the pathogenicity. In conclusion, congenital CV-A6 infection should be considered if a woman exhibits HFMD symptoms during the perinatal period. Detailed virologic examination is useful for understanding its pathogenesis.
Keywords: Coxsackievirus A6; HFMD; congenital pneumonia; multiplex PCR; vertical transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wang S.H., Wang A., Liu P.P., Zhang W.Y., Du J., Xu S., Liu G.C., Zheng B.S., Huan C., Zhao K., et al. Divergent Pathogenic Properties of Circulating Coxsackievirus A6 Associated with Emerging Hand, Foot, and Mouth Disease. J. Virol. 2018;92:e00303–e00318. doi: 10.1128/JVI.00303-18. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

