COBL, MKX and MYOC Are Potential Regulators of Brown Adipose Tissue Development Associated with Obesity-Related Metabolic Dysfunction in Children
- PMID: 36834493
- PMCID: PMC9964948
- DOI: 10.3390/ijms24043085
COBL, MKX and MYOC Are Potential Regulators of Brown Adipose Tissue Development Associated with Obesity-Related Metabolic Dysfunction in Children
Abstract
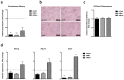
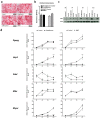
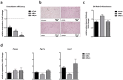
Obesity is already accompanied by adipose tissue (AT) dysfunction and metabolic disease in children and increases the risk of premature death. Due to its energy-dissipating function, brown AT (BAT) has been discussed as being protective against obesity and related metabolic dysfunction. To analyze the molecular processes associated with BAT development, we investigated genome-wide expression profiles in brown and white subcutaneous and perirenal AT samples of children. We identified 39 upregulated and 26 downregulated genes in uncoupling protein 1 (UCP1)-positive compared to UCP1-negative AT samples. We prioritized for genes that had not been characterized regarding a role in BAT biology before and selected cordon-bleu WH2 repeat protein (COBL), mohawk homeobox (MKX) and myocilin (MYOC) for further functional characterization. The siRNA-mediated knockdown of Cobl and Mkx during brown adipocyte differentiation in vitro resulted in decreased Ucp1 expression, while the inhibition of Myoc led to increased Ucp1 expression. Furthermore, COBL, MKX and MYOC expression in the subcutaneous AT of children is related to obesity and parameters of AT dysfunction and metabolic disease, such as adipocyte size, leptin levels and HOMA-IR. In conclusion, we identify COBL, MKX and MYOC as potential regulators of BAT development and show an association of these genes with early metabolic dysfunction in children.
Keywords: COBL; MKX; MYOC; adipocyte differentiation; adipose tissue; brown adipose tissue; children; metabolic disease; obesity; white adipose tissue.
Conflict of interest statement
The authors do not claim any conflict of interest or competing financial interest.
Figures




Similar articles
-
Standardized In Vitro Models of Human Adipose Tissue Reveal Metabolic Flexibility in Brown Adipocyte Thermogenesis.Endocrinology. 2023 Nov 2;164(12):bqad161. doi: 10.1210/endocr/bqad161. Endocrinology. 2023. PMID: 37944134 Free PMC article.
-
Transcriptomic Analysis Reveals Fibroblast Growth Factor 11 (FGF11) Role in Brown Adipocytes in Thermogenic Regulation of Goats.Int J Mol Sci. 2023 Jun 29;24(13):10838. doi: 10.3390/ijms241310838. Int J Mol Sci. 2023. PMID: 37446019 Free PMC article.
-
Differentiation and characterization in primary culture of white adipose tissue brown adipocyte-like cells.Int J Obes (Lond). 2009 Jun;33(6):680-6. doi: 10.1038/ijo.2009.46. Epub 2009 Mar 10. Int J Obes (Lond). 2009. PMID: 19274054
-
Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity.Biochimie. 2017 Mar;134:62-70. doi: 10.1016/j.biochi.2016.09.007. Epub 2016 Sep 10. Biochimie. 2017. PMID: 27621146 Review.
-
Mitochondrial Energy Metabolism in the Regulation of Thermogenic Brown Fats and Human Metabolic Diseases.Int J Mol Sci. 2023 Jan 10;24(2):1352. doi: 10.3390/ijms24021352. Int J Mol Sci. 2023. PMID: 36674862 Free PMC article. Review.
Cited by
-
Uncovering the candidate genes related to sheep body weight using multi-trait genome-wide association analysis.Front Vet Sci. 2023 Aug 17;10:1206383. doi: 10.3389/fvets.2023.1206383. eCollection 2023. Front Vet Sci. 2023. PMID: 37662987 Free PMC article.
-
Transcriptomic profiling of backfat and muscle in Lijiang pigs with divergent body size across growth stages.Sci Rep. 2024 Nov 12;14(1):27677. doi: 10.1038/s41598-024-78054-6. Sci Rep. 2024. PMID: 39532937 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

