Nrf2 and Antioxidant Response in Animal Models of Type 2 Diabetes
- PMID: 36834496
- PMCID: PMC9961396
- DOI: 10.3390/ijms24043082
Nrf2 and Antioxidant Response in Animal Models of Type 2 Diabetes
Abstract
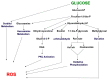
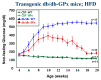
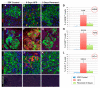
This perspective examines the proposition that chronically elevated blood glucose levels caused by type 2 diabetes (T2D) harm body tissues by locally generating reactive oxygen species (ROS). A feed-forward scenario is described in which the initial onset of defective beta cell function T2D becomes sustained and causes chronic elevations in blood glucose, which flood metabolic pathways throughout the body, giving rise to abnormally high local levels of ROS. Most cells can defend themselves via a full complement of antioxidant enzymes that are activated by ROS. However, the beta cell itself does not contain catalase or glutathione peroxidases and thereby runs a greater risk of ROS-induced damage. In this review, previously published experiments are revisited to examine the concept that chronic hyperglycemia can lead to oxidative stress in the beta cell, how this relates to the absence of beta cell glutathione peroxidase (GPx) activity, and whether this deficiency might be ameliorated by genetic enrichment of beta cell GPx and by oral antioxidants, including ebselen, a GPx mimetic.
Keywords: Nrf2; antioxidant response; type 2 diabetes in animals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Takahashi H., Tran P.O.T., LeRoy E., Harmon J., Tanaka Y., Robertson R.P. D-glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective beta cell function via non-mitochondrial pathways. J. Biol. Chem. 2004;279:37316–37323. doi: 10.1074/jbc.M403070200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

