Syndecan-4 Mediates the Cellular Entry of Adeno-Associated Virus 9
- PMID: 36834552
- PMCID: PMC9963952
- DOI: 10.3390/ijms24043141
Syndecan-4 Mediates the Cellular Entry of Adeno-Associated Virus 9
Abstract
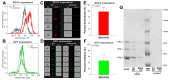
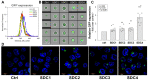
Due to their low pathogenicity, immunogenicity, and long-term gene expression, adeno-associated virus (AAV) vectors emerged as safe and efficient gene delivery tools, over-coming setbacks experienced with other viral gene delivery systems in early gene therapy trials. Among AAVs, AAV9 can translocate through the blood-brain barrier (BBB), making it a promising gene delivery tool for transducing the central nervous system (CNS) via systemic administration. Recent reports on the shortcomings of AAV9-mediated gene delivery into the CNS require reviewing the molecular base of AAV9 cellular biology. A more detailed understanding of AAV9's cellular entry would eradicate current hurdles and enable more efficient AAV9-based gene therapy approaches. Syndecans, the transmembrane family of heparan-sulfate proteoglycans, facilitate the cellular uptake of various viruses and drug delivery systems. Utilizing human cell lines and syndecan-specific cellular assays, we assessed the involvement of syndecans in AAV9's cellular entry. The ubiquitously expressed isoform, syndecan-4 proved its superiority in facilitating AAV9 internalization among syndecans. Introducing syndecan-4 into poorly transducible cell lines enabled robust AAV9-dependent gene transduction, while its knockdown reduced AAV9's cellular entry. Attachment of AAV9 to syndecan-4 is mediated not just by the polyanionic heparan-sulfate chains but also by the cell-binding domain of the extracellular syndecan-4 core protein. Co-immunoprecipitation assays and affinity proteomics also confirmed the role of syndecan-4 in the cellular entry of AAV9. Overall, our findings highlight the universally expressed syndecan-4 as a significant contributor to the cellular internalization of AAV9 and provide a molecular-based, rational explanation for the low gene delivery potential of AAV9 into the CNS.
Keywords: AAV9; cellular entry; gene delivery; heparan sulfate proteoglycans; syndecans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Misra S. Human gene therapy: A brief overview of the genetic revolution. J. Assoc. Physicians India. 2013;61:127–133. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

