Unraveling the Potential Role of Tecomella undulata in Experimental NASH
- PMID: 36834657
- PMCID: PMC9962064
- DOI: 10.3390/ijms24043244
Unraveling the Potential Role of Tecomella undulata in Experimental NASH
Abstract
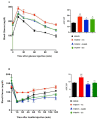
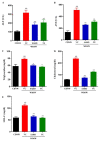
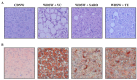
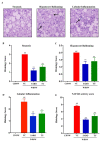
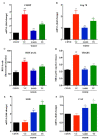
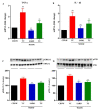
The pathophysiology of nonalcoholic steatohepatitis (NASH) is complex, owing to its diverse pathological drivers and, until recently, there were no approved drugs for this disease. Tecomella is a popular herbal medicine used to treat hepatosplenomegaly, hepatitis, and obesity. However, the potential role of Tecomella undulata in NASH has not yet been scientifically investigated. The administration of Tecomella undulata via oral gavage lowered body weight, insulin resistance, alanine transaminase (ALT), aspartate transaminase (AST), triglycerides, and total cholesterol in western diet sugar water (WDSW) fed mice but had no effect on chow diet normal water (CDNW) fed mice. Tecomella undulata improved steatosis, lobular inflammation, and hepatocyte ballooning and resolved NASH in WDSW mice. Furthermore, Tecomella undulata also alleviated the WDSW-induced Endoplasmic Reticulum stress and oxidative stress, enhanced antioxidant status, and thus reduced inflammation in the treated mice. Of note, these effects were comparable to saroglitazar, the approved drug used to treat human NASH and the positive control used in the study. Thus, our findings indicate the potential of Tecomella undulata to ameliorate WDSW-induced steatohepatitis, and these preclinical data provide a strong rationale for assessing Tecomella undulata for the treatment of NASH.
Keywords: ER stress; hepatic inflammation; herbal medicine; insulin resistance; oxidative stress.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

