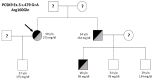
Functional Characterization of p.(Arg160Gln) PCSK9 Variant Accidentally Found in a Hypercholesterolemic Subject
- PMID: 36834740
- PMCID: PMC9959173
- DOI: 10.3390/ijms24043330
Functional Characterization of p.(Arg160Gln) PCSK9 Variant Accidentally Found in a Hypercholesterolemic Subject
Abstract
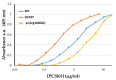
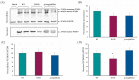
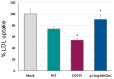
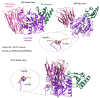
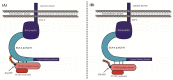
Familial hypercholesterolaemia (FH) is an autosomal dominant dyslipidaemia, characterised by elevated LDL cholesterol (LDL-C) levels in the blood. Three main genes are involved in FH diagnosis: LDL receptor (LDLr), Apolipoprotein B (APOB) and Protein convertase subtilisin/kexin type 9 (PCSK9) with genetic mutations that led to reduced plasma LDL-C clearance. To date, several PCSK9 gain-of-function (GOF) variants causing FH have been described based on their increased ability to degrade LDLr. On the other hand, mutations that reduce the activity of PCSK9 on LDLr degradation have been described as loss-of-function (LOF) variants. It is therefore important to functionally characterise PCSK9 variants in order to support the genetic diagnosis of FH. The aim of this work is to functionally characterise the p.(Arg160Gln) PCSK9 variant found in a subject suspected to have FH. Different techniques have been combined to determine efficiency of the autocatalytic cleavage, protein expression, effect of the variant on LDLr activity and affinity of the PCSK9 variant for the LDLr. Expression and processing of the p.(Arg160Gln) variant had a result similar to that of WT PCSK9. The effect of p.(Arg160Gln) PCSK9 on LDLr activity is lower than WT PCSK9, with higher values of LDL internalisation (13%) and p.(Arg160Gln) PCSK9 affinity for the LDLr is lower than WT, EC50 8.6 ± 0.8 and 25.9 ± 0.7, respectively. The p.(Arg160Gln) PCSK9 variant is a LOF PCSK9 whose loss of activity is caused by a displacement of the PCSK9 P' helix, which reduces the stability of the LDLr-PCSK9 complex.
Keywords: GOF; LOF; PCSK9; activity; characterisation; familial hypercholesterolemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

