Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery
- PMID: 36834823
- PMCID: PMC9963055
- DOI: 10.3390/ijms24043412
Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery
Abstract
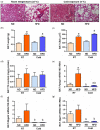
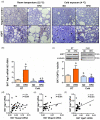
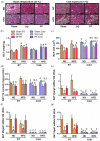
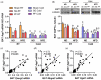
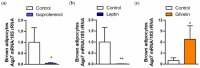
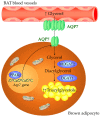
Glycerol is a key metabolite for lipid accumulation in insulin-sensitive tissues. We examined the role of aquaporin-7 (AQP7), the main glycerol channel in adipocytes, in the improvement of brown adipose tissue (BAT) whitening, a process whereby brown adipocytes differentiate into white-like unilocular cells, after cold exposure or bariatric surgery in male Wistar rats with diet-induced obesity (DIO) (n = 229). DIO promoted BAT whitening, evidenced by increased BAT hypertrophy, steatosis and upregulation of the lipogenic factors Pparg2, Mogat2 and Dgat1. AQP7 was detected in BAT capillary endothelial cells and brown adipocytes, and its expression was upregulated by DIO. Interestingly, AQP7 gene and protein expressions were downregulated after cold exposure (4 °C) for 1 week or one month after sleeve gastrectomy in parallel to the improvement of BAT whitening. Moreover, Aqp7 mRNA expression was positively associated with transcripts of the lipogenic factors Pparg2, Mogat2 and Dgat1 and regulated by lipogenic (ghrelin) and lipolytic (isoproterenol and leptin) signals. Together, the upregulation of AQP7 in DIO might contribute to glycerol influx used for triacylglycerol synthesis in brown adipocytes, and hence, BAT whitening. This process is reversible by cold exposure and bariatric surgery, thereby suggesting the potential of targeting BAT AQP7 as an anti-obesity therapy.
Keywords: adipocyte hypertrophy; aquaglyceroporin; lipogenesis; peroxisome proliferator-activated receptor γ; sleeve gastrectomy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Frühbeck G., Busetto L., Dicker D., Yumuk V., Goossens G.H., Hebebrand J., Halford J.G.C., Farpour-Lambert N.J., Blaak E.E., Woodward E., et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts. 2019;12:131–136. doi: 10.1159/000497124. - DOI - PMC - PubMed
-
- Kotzbeck P., Giordano A., Mondini E., Murano I., Severi I., Venema W., Cecchini M.P., Kershaw E.E., Barbatelli G., Haemmerle G., et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018;59:784–794. doi: 10.1194/jlr.M079665. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

