Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1, 3, 4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies
- PMID: 36834848
- PMCID: PMC9966784
- DOI: 10.3390/ijms24043430
Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1, 3, 4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies
Abstract
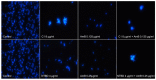
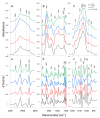
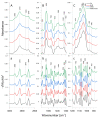
In recent years, drug-resistant and multidrug-resistant fungal strains have been more frequently isolated in clinical practice. This phenomenon is responsible for difficulties in the treatment of infections. Therefore, the development of new antifungal drugs is an extremely important challenge. Combinations of selected 1,3,4-thiadiazole derivatives with amphotericin B showing strong synergic antifungal interactions are promising candidates for such formulas. In the study, microbiological, cytochemical, and molecular spectroscopy methods were used to investigate the antifungal synergy mechanisms associated with the aforementioned combinations. The present results indicate that two derivatives, i.e., C1 and NTBD, demonstrate strong synergistic interactions with AmB against some Candida species. The ATR-FTIR analysis showed that yeasts treated with the C1 + AmB and NTBD + AmB compositions, compared with those treated with single compounds, exhibited more pronounced abnormalities in the biomolecular content, suggesting that the main mechanism of the synergistic antifungal activity of the compounds is related to a disturbance in cell wall integrity. The analysis of the electron absorption and fluorescence spectra revealed that the biophysical mechanism underlying the observed synergy is associated with disaggregation of AmB molecules induced by the 1,3,4-thiadiazole derivatives. Such observations suggest the possibility of the successful application of thiadiazole derivatives combined with AmB in the therapy of fungal infections.
Keywords: 1,3,4-thiadiazole derivatives; FTIR spectroscopy; amphotericin B; molecular aggregation; molecular spectroscopy; synergy mechanisms.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

