The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy
- PMID: 36835043
- PMCID: PMC9964330
- DOI: 10.3390/ijms24043632
The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy
Abstract
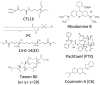
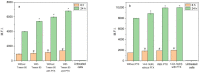
Cerasomes are a promising modification of liposomes with covalent siloxane networks on the surface that provide outstanding morphological stability while maintaining all the useful traits of liposomes. Herein, thin film hydration and ethanol sol injection methods were utilized to produce cerasomes of various composition, which were then evaluated for the purpose of drug delivery. The most promising nanoparticles obtained by the thin film method were studied closely using MTT assay, flow cytometry and fluorescence microscopy on T98G glioblastoma cell line and modified with surfactants to achieve stability and the ability to bypass the blood-brain barrier. An antitumor agent, paclitaxel, was loaded into cerasomes, which increased its potency and demonstrated increased ability to induce apoptosis in T98G glioblastoma cell culture. Cerasomes loaded with fluorescent dye rhodamine B demonstrated significantly increased fluorescence in brain slices of Wistar rats compared to free rhodamine B. Thin film hydration with Tween 80 addition was established as a more reliable and versatile method for cerasome preparation. Cerasomes increased the antitumor action of paclitaxel toward T98G cancer cells by a factor of 36 and were able to deliver rhodamine B over the blood-brain barrier in rats.
Keywords: T98G cells; Tween 80; blood–brain barrier; cerasome; cytotoxicity; liposome; paclitaxel; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

