Anti-Atherogenic Actions of the Lab4b Consortium of Probiotics In Vitro
- PMID: 36835055
- PMCID: PMC9964490
- DOI: 10.3390/ijms24043639
Anti-Atherogenic Actions of the Lab4b Consortium of Probiotics In Vitro
Abstract
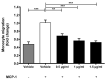
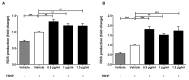
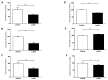
Probiotic bacteria have many protective effects against inflammatory disorders, though the mechanisms underlying their actions are poorly understood. The Lab4b consortium of probiotics contains four strains of lactic acid bacteria and bifidobacteria that are reflective of the gut of newborn babies and infants. The effect of Lab4b on atherosclerosis, an inflammatory disorder of the vasculature, has not yet been determined and was investigated on key processes associated with this disease in human monocytes/macrophages and vascular smooth muscle cells in vitro. The Lab4b conditioned medium (CM) attenuated chemokine-driven monocytic migration, monocyte/macrophage proliferation, uptake of modified LDL and macropinocytosis in macrophages together with the proliferation and platelet-derived growth factor-induced migration of vascular smooth muscle cells. The Lab4b CM also induced phagocytosis in macrophages and cholesterol efflux from macrophage-derived foam cells. The effect of Lab4b CM on macrophage foam cell formation was associated with a decrease in the expression of several key genes implicated in the uptake of modified LDL and induced expression of those involved in cholesterol efflux. These studies reveal, for the first time, several anti-atherogenic actions of Lab4b and strongly implicate further studies in mouse models of the disease in vivo and in clinical trials.
Keywords: atherosclerosis; foam cells; gene expression; inflammation; macrophages; probiotics; smooth muscle cells.
Conflict of interest statement
This study was supported in part by Cultech Ltd., Port Talbot, UK. D.R.M. and S.F.P. are employees of Cultech Ltd. V.L.M. was funded by a Knowledge Economy Skills Scholarship with Cultech Ltd.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

