Autophagy and the Insulin-like Growth Factor (IGF) System in Colonic Cells: Implications for Colorectal Neoplasia
- PMID: 36835075
- PMCID: PMC9959216
- DOI: 10.3390/ijms24043665
Autophagy and the Insulin-like Growth Factor (IGF) System in Colonic Cells: Implications for Colorectal Neoplasia
Abstract
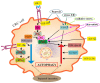
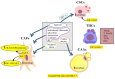
Colorectal cancer (CRC) is one of the most common human malignancies worldwide. Along with apoptosis and inflammation, autophagy is one of three important mechanisms in CRC. The presence of autophagy/mitophagy in most normal mature intestinal epithelial cells has been confirmed, where it has mainly protective functions against reactive oxygen species (ROS)-induced DNA and protein damage. Autophagy regulates cell proliferation, metabolism, differentiation, secretion of mucins and/or anti-microbial peptides. Abnormal autophagy in intestinal epithelial cells leads to dysbiosis, a decline in local immunity and a decrease in cell secretory function. The insulin-like growth factor (IGF) signaling pathway plays an important role in colorectal carcinogenesis. This is evidenced by the biological activities of IGFs (IGF-1 and IGF-2), IGF-1 receptor type 1 (IGF-1R) and IGF-binding proteins (IGF BPs), which have been reported to regulate cell survival, proliferation, differentiation and apoptosis. Defects in autophagy are found in patients with metabolic syndrome (MetS), inflammatory bowel diseases (IBD) and CRC. In neoplastic cells, the IGF system modulates the autophagy process bidirectionally. In the current era of improving CRC therapies, it seems important to investigate the exact mechanisms not only of apoptosis, but also of autophagy in different populations of tumor microenvironment (TME) cells. The role of the IGF system in autophagy in normal as well as transformed colorectal cells still seems poorly understood. Hence, the aim of the review was to summarize the latest knowledge on the role of the IGF system in the molecular mechanisms of autophagy in the normal colon mucosa and in CRC, taking into account the cellular heterogeneity of the colonic and rectal epithelium.
Keywords: autophagy; colonic and rectal epithelial cells; colorectal cancer; insulin-like growth factor (IGF) system; tumor microenvironment (TME) cells.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

