Environmental Enrichment Promotes Transgenerational Programming of Uterine Inflammatory and Stress Markers Comparable to Gestational Chronic Variable Stress
- PMID: 36835144
- PMCID: PMC9962069
- DOI: 10.3390/ijms24043734
Environmental Enrichment Promotes Transgenerational Programming of Uterine Inflammatory and Stress Markers Comparable to Gestational Chronic Variable Stress
Abstract
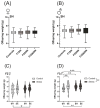
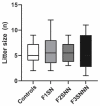
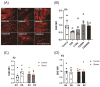
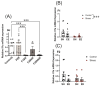
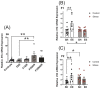
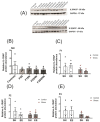
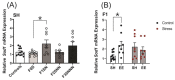
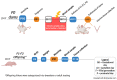
Prenatal maternal stress is linked to adverse pregnancy and infant outcomes, including shortened gestation lengths, low birth weights, cardio-metabolic dysfunction, and cognitive and behavioural problems. Stress disrupts the homeostatic milieu of pregnancy by altering inflammatory and neuroendocrine mediators. These stress-induced phenotypic changes can be passed on to the offspring epigenetically. We investigated the effects of gestational chronic variable stress (CVS) in rats using restraint and social isolation stress in the parental F0 generation and its transgenerational transmission across three generations of female offspring (F1-F3). A subset of F1 rats was housed in an enriched environment (EE) to mitigate the adverse effects of CVS. We found that CVS is transmitted across generations and induces inflammatory changes in the uterus. CVS did not alter any gestational lengths or birth weights. However, inflammatory and endocrine markers changed in the uterine tissues of stressed mothers and their offspring, suggesting that stress is transgenerationally transmitted. The F2 offspring reared in EE had increased birth weights, but their uterine gene expression patterns remained comparable to those of stressed animals. Thus, ancestral CVS induced changes transgenerationally in fetal programming of uterine stress markers over three generations of offspring, and EE housing did not mitigate these effects.
Keywords: chronic variable stress; enriched environment; gene expression; inflammation; pregnancy; prenatal stress; preterm birth; resilience; rodents; uterus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Yao Y., Robinson A.M., Zucchi F.C., Robbins J.C., Babenko O., Kovalchuk O., Kovalchuk I., Olson D.M., Metz G.A. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med. 2014;12:121. doi: 10.1186/s12916-014-0121-6. - DOI - PMC - PubMed
-
- Garcia-Flores V., Romero R., Furcron A.E., Levenson D., Galaz J., Zou C., Hassan S.S., Hsu C.D., Olson D., Metz G.A.S., et al. Prenatal Maternal Stress Causes Preterm Birth and Affects Neonatal Adaptive Immunity in Mice. Front. Immunol. 2020;11:254. doi: 10.3389/fimmu.2020.00254. - DOI - PMC - PubMed
-
- Chen H.J., Antonson A.M., Rajasekera T.A., Patterson J.M., Bailey M.T., Gur T.L. Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl. Psychiatry. 2020;10:191. doi: 10.1038/s41398-020-00876-5. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

