First X-ray Crystal Structure Characterization, Computational Studies, and Improved Synthetic Route to the Bioactive 5-Arylimino-1,3,4-thiadiazole Derivatives
- PMID: 36835167
- PMCID: PMC9965731
- DOI: 10.3390/ijms24043759
First X-ray Crystal Structure Characterization, Computational Studies, and Improved Synthetic Route to the Bioactive 5-Arylimino-1,3,4-thiadiazole Derivatives
Abstract
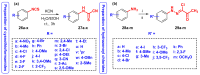
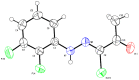
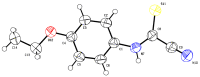
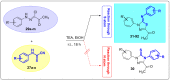
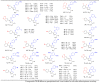
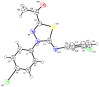
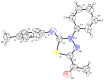
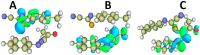
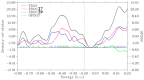
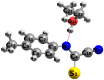
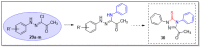
N-arylcyanothioformamides are useful coupling components for building key chemical intermediates and biologically active molecules in an expedited and efficient manner. Similarly, substituted (Z)-2-oxo-N-phenylpropanehydrazonoyl chlorides have been utilized in numerous one-step heteroannulation reactions to assemble the structural core of several different types of heterocyclic compounds. Herein, we demonstrate the effectiveness of the reaction of N-arylcyanothioformamides with various substituted (Z)-2-oxo-N-phenylpropanehydrazonoyl chlorides to produce, stereoselectively and regioselectively, a range of 5-arylimino-1,3,4-thiadiazole derivatives decorated with a multitude of functional groups on both aromatic rings. The synthetic methodology features mild room-temperature conditions, large substrate scope, wide array of functional groups on both reactants, and good to high reaction yields. The products were isolated by gravity filtration in all cases and structures were confirmed by multinuclear NMR spectroscopy and high accuracy mass spectral analysis. Proof of molecular structure of the isolated 5-arylimino-1,3,4-thiadiazole regioisomer was obtained for the first time by single-crystal X-ray diffraction analysis. Crystal-structure determination was carried out on (Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one and (Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one. Similarly, the tautomeric structures of the N-arylcyanothioformamides and (Z)-geometries of the 2-oxo-N-phenylpropanehydrazonoyl chloride coupling partners were proven by X-ray diffraction studies. As representative examples, crystal-structure determination was carried out on (4-ethoxyphenyl)carbamothioyl cyanide and (Z)-N-(2,3-difluorophenyl)-2-oxopropanehydrazonoyl chloride. Density functional theory calculations at the B3LYP-D4/def2-TZVP level were carried out to rationalize the observed experimental findings.
Keywords: 1,3,4-thiadiazole; N-arylcyanothioformamide; density functional theory; hydrazonoyl chloride; isothiocyanate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zoumpoulakis P., Camoutsis C., Pairas G., Sokovic M., Glamoclija J., Potamitis C., Pitsas A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012;20:1569–1583. doi: 10.1016/j.bmc.2011.12.031. - DOI - PubMed
-
- Hasui T., Matsunaga N., Ora T., Ohyabu N., Nishigaki N., Imura Y., Igata Y., Matsui H., Motoyaji T., Tanaka T., et al. Identification of benzoxazin-3-one derivatives as novel, potent, and selective nonsteroidal mineralocorticoid receptor antagonists. Med. Chem. 2011;54:8616–8631. doi: 10.1021/jm2011645. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

