The Role of the Heat Shock Cognate Protein 70 Genes in Sex Determination and Differentiation of Chinese Tongue Sole (Cynoglossus semilaevis)
- PMID: 36835170
- PMCID: PMC9964925
- DOI: 10.3390/ijms24043761
The Role of the Heat Shock Cognate Protein 70 Genes in Sex Determination and Differentiation of Chinese Tongue Sole (Cynoglossus semilaevis)
Abstract
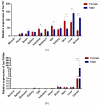
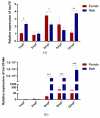
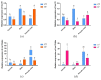
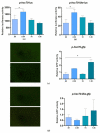
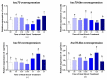
Fish sex determination can be affected by environmental temperature. This process relies on temperature-sensitive proteins such as heat shock proteins (HSPs). Our previous work found that heat shock cognate proteins (HSCs) may participate in high-temperature associated sex reversal of Chinese tongue sole (Cynoglossus semilaevis). However, the role of hsc genes in responding to high temperature and affecting sex determination/differentiation remains unclear. Here, by using C. semilaevis as model, we identified hsc70 and hsc70-like. hsc70 was abundant in the gonads with a testicular-higher expression at all gonadal development stages except for 6 months post fertilization (mpf). Intriguingly, hsc70-like showed higher expression in testes from 6 mpf on. Both long-term heat treatment during the temperature-sensitive sex-determining period and short-term heat stress at the end of this period caused different expression of hsc70/hsc70-like between sexes. The dual-luciferase assay results also suggested that these genes can respond to high temperature rapidly in vitro. Heat treatment of C. semilaevis testis cells overexpressed with hsc70/hsc70-like could affect the expression of sex-related genes sox9a and cyp19a1a. Our results indicated that hsc70 and hsc70-like were key regulators linking external high-temperature signals with sex differentiation in vivo and provide a new idea for understanding the mechanism by which high temperature affects sex determination/differentiation in teleosts.
Keywords: Cynoglossus semilaevis; high temperature; hsc70 genes; sex determination and differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bull J.J. Evolution of environmental sex determination from genotypic sex determination. Heredity. 1981;47:173–184. doi: 10.1038/hdy.1981.74. - DOI
-
- Desprez D., Mélard C. Effect of ambient water temperature on sex determinism in the blue tilapia Oreochromis aureus. Aquaculture. 1998;162:79–84. doi: 10.1016/S0044-8486(97)00242-1. - DOI
MeSH terms
Substances
Grants and funding
- 2018YFD0900301/the National Key R&D Program of China
- 31722058/the National Nature Science Foundation of China
- 31472269/the National Nature Science Foundation of China
- 20603022021018/the Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS
- 2017ASTCP-ES06/the AoShan Talents Cultivation Program Supported by Qingdao National Laboratory for Marine Science and Technology
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

