Tuning the Wavelength: Manipulation of Light Signaling to Control Plant Defense
- PMID: 36835216
- PMCID: PMC9958957
- DOI: 10.3390/ijms24043803
Tuning the Wavelength: Manipulation of Light Signaling to Control Plant Defense
Abstract
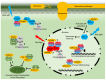
The growth-defense trade-off in plants is a phenomenon whereby plants must balance the allocation of their resources between developmental growth and defense against attack by pests and pathogens. Consequently, there are a series of points where growth signaling can negatively regulate defenses and where defense signaling can inhibit growth. Light perception by various photoreceptors has a major role in the control of growth and thus many points where it can influence defense. Plant pathogens secrete effector proteins to manipulate defense signaling in their hosts. Evidence is emerging that some of these effectors target light signaling pathways. Several effectors from different kingdoms of life have converged on key chloroplast processes to take advantage of regulatory crosstalk. Moreover, plant pathogens also perceive and react to light in complex ways to regulate their own growth, development, and virulence. Recent work has shown that varying light wavelengths may provide a novel way of controlling or preventing disease outbreaks in plants.
Keywords: immunity; light; pathogen effectors; pathogenicity; plant; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fernández-Milmanda G.L., Crocco C.D., Reichelt M., Mazza C.A., Köllner T.G., Zhang T., Cargnel M.D., Lichy M.Z., Fiorucci A.S., Fankhauser C., et al. A Light-Dependent Molecular Link between Competition Cues and Defence Responses in Plants. Nat. Plants. 2020;6:223–230. doi: 10.1038/s41477-020-0604-8. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

