Characterization of the Antitumor Potential of Extracts of Cannabis sativa Strains with High CBD Content in Human Neuroblastoma
- PMID: 36835247
- PMCID: PMC9964014
- DOI: 10.3390/ijms24043837
Characterization of the Antitumor Potential of Extracts of Cannabis sativa Strains with High CBD Content in Human Neuroblastoma
Abstract
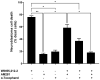
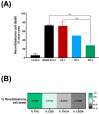
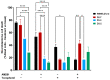
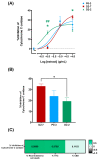
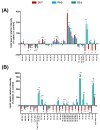
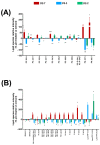
Cannabis has been used for decades as a palliative therapy in the treatment of cancer. This is because of its beneficial effects on the pain and nausea that patients can experience as a result of chemo/radiotherapy. Tetrahydrocannabinol and cannabidiol are the main compounds present in Cannabis sativa, and both exert their actions through a receptor-mediated mechanism and through a non-receptor-mediated mechanism, which modulates the formation of reactive oxygen species. These oxidative stress conditions might trigger lipidic changes, which would compromise cell membrane stability and viability. In this sense, numerous pieces of evidence describe a potential antitumor effect of cannabinoid compounds in different types of cancer, although controversial results limit their implementation. In order to further investigate the possible mechanism involved in the antitumoral effects of cannabinoids, three extracts isolated from Cannabis sativa strains with high cannabidiol content were analyzed. Cell mortality, cytochrome c oxidase activity and the lipid composition of SH-SY5Y cells were determined in the absence and presence of specific cannabinoid ligands, with and without antioxidant pre-treatment. The cell mortality induced by the extracts in this study appeared to be related to the inhibition of the cytochrome c oxidase activity and to the THC concentration. This effect on cell viability was similar to that observed with the cannabinoid agonist WIN55,212-2. The effect was partially blocked by the selective CB1 antagonist AM281, and the antioxidant α-tocopherol. Moreover, certain membrane lipids were affected by the extracts, which demonstrated the importance of oxidative stress in the potential antitumoral effects of cannabinoids.
Keywords: antitumor; cannabis; extracts; neuroblastoma.
Conflict of interest statement
All co-authors from IMG Pharma Biotech S.L. have no conflict of interest in regard to the present study for publication. E.A. and G.B.-G. are listed as inventors on a patent (EP2048534A4). The remaining authors declare no conflict of interest.
Figures
Similar articles
-
Screening System of Cannabis sativa Extracts Based on Their Mitochondrial Safety Profile Using Cytochrome c Oxidase Activity as a Biomarker.Int J Mol Sci. 2023 Jan 10;24(2):1315. doi: 10.3390/ijms24021315. Int J Mol Sci. 2023. PMID: 36674832 Free PMC article.
-
¹H NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification.Talanta. 2015 Aug 1;140:150-165. doi: 10.1016/j.talanta.2015.02.040. Epub 2015 Mar 5. Talanta. 2015. PMID: 26048837
-
Analysis of Anti-Cancer and Anti-Inflammatory Properties of 25 High-THC Cannabis Extracts.Molecules. 2022 Sep 16;27(18):6057. doi: 10.3390/molecules27186057. Molecules. 2022. PMID: 36144796 Free PMC article.
-
Constituents of Cannabis Sativa.Adv Exp Med Biol. 2021;1264:1-13. doi: 10.1007/978-3-030-57369-0_1. Adv Exp Med Biol. 2021. PMID: 33332000 Review.
-
Using the BMD Approach to Derive Acceptable Daily Intakes of Cannabidiol (CBD) and Tetrahydrocannabinol (THC) Relevant to Electronic Cigarette Liquids.Front Biosci (Landmark Ed). 2022 Jul 25;27(8):228. doi: 10.31083/j.fbl2708228. Front Biosci (Landmark Ed). 2022. PMID: 36042166
Cited by
-
Cannabinoids in Medicine: A Multifaceted Exploration of Types, Therapeutic Applications, and Emerging Opportunities in Neurodegenerative Diseases and Cancer Therapy.Biomolecules. 2023 Sep 14;13(9):1388. doi: 10.3390/biom13091388. Biomolecules. 2023. PMID: 37759788 Free PMC article. Review.
-
New Cannabinoids and Chlorin-Type Metabolites from the Flowers of Cannabis sativa L.: A Study on Their Neuroblastoma Activity.Pharmaceuticals (Basel). 2025 Apr 3;18(4):521. doi: 10.3390/ph18040521. Pharmaceuticals (Basel). 2025. PMID: 40283956 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

