Dipotassium Glycyrrhizininate Improves Skin Wound Healing by Modulating Inflammatory Process
- PMID: 36835248
- PMCID: PMC9965141
- DOI: 10.3390/ijms24043839
Dipotassium Glycyrrhizininate Improves Skin Wound Healing by Modulating Inflammatory Process
Abstract
Wound healing is characterized by a systemic and complex process of cellular and molecular activities. Dipotassium Glycyrrhizinate (DPG), a side product derived from glycyrrhizic acid, has several biological effects, such as being antiallergic, antioxidant, antibacterial, antiviral, gastroprotective, antitumoral, and anti-inflammatory. This study aimed to evaluate the anti-inflammatory effect of topical DPG on the healing of cutaneous wounds by secondary intention in an in vivo experimental model. Twenty-four male Wistar rats were used in the experiment, and were randomly divided into six groups of four. Circular excisions were performed and topically treated for 14 days after wound induction. Macroscopic and histopathological analyses were performed. Gene expression was evaluated by real-time qPCR. Our results showed that treatment with DPG caused a decrease in the inflammatory exudate as well as an absence of active hyperemia. Increases in granulation tissue, tissue reepithelization, and total collagen were also observed. Furthermore, DPG treatment reduced the expression of pro-inflammatory cytokines (Tnf-α, Cox-2, Il-8, Irak-2, Nf-kB, and Il-1) while increasing the expression of Il-10, demonstrating anti-inflammatory effects across all three treatment periods. Based on our results, we conclude that DPG attenuates the inflammatory process by promoting skin wound healing through the modulation of distinct mechanisms and signaling pathways, including anti-inflammatory ones. This involves modulation of the expression of pro- and anti-inflammatory cytokine expression; promotion of new granulation tissue; angiogenesis; and tissue re-epithelialization, all of which contribute to tissue remodeling.
Keywords: DPG; animal model; inflammation; rats; skin wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
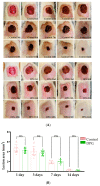



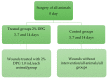
References
-
- Scully D., Sfyri P., Wilkinson H.N., Acebes-Huerta A., Verpoorten S., Muñoz-Turrillas M.C., Parnell A., Patel K., Hardman M.J., Gutiérrez L., et al. Optimising platelet secretomes to deliver robust tissue-specific regeneration. J. Tissue Eng. Regen. Med. 2020;14:82–98. doi: 10.1002/term.2965. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

