Biophysical Parameters Can Induce Epithelial-to-Mesenchymal Phenotypic and Genotypic Changes in HT-29 Cells: A Preliminary Study
- PMID: 36835368
- PMCID: PMC9962772
- DOI: 10.3390/ijms24043956
Biophysical Parameters Can Induce Epithelial-to-Mesenchymal Phenotypic and Genotypic Changes in HT-29 Cells: A Preliminary Study
Abstract
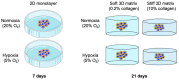
Epithelial to mesenchymal transition (EMT) in cancer is the process described where cancer epithelial cells acquire mesenchymal properties which can lead to enhanced invasiveness. Three-dimensional cancer models often lack the relevant and biomimetic microenvironment parameters appropriate to the native tumour microenvironment thought to drive EMT. In this study, HT-29 epithelial colorectal cells were cultivated in different oxygen and collagen concentrations to investigate how these biophysical parameters influenced invasion patterns and EMT. Colorectal HT-29 cells were grown in physiological hypoxia (5% O2) and normoxia (21% O2) in 2D, 3D soft (60 Pa), and 3D stiff (4 kPa) collagen matrices. Physiological hypoxia was sufficient to trigger expression of markers of EMT in the HT-29 cells in 2D by day 7. This is in contrast to a control breast cancer cell line, MDA-MB-231, which expresses a mesenchymal phenotype regardless of the oxygen concentration. In 3D, HT-29 cells invaded more extensively in a stiff matrix environment with corresponding increases in the invasive genes MMP2 and RAE1. This demonstrates that the physiological environment can directly impact HT-29 cells in terms of EMT marker expression and invasion, compared to an established cell line, MDA-MB-231, which has already undergone EMT. This study highlights the importance of the biophysical microenvironment to cancer epithelial cells and how these factors can direct cell behaviour. In particular, that stiffness of the 3D matrix drives greater invasion in HT-29 cells regardless of hypoxia. It is also pertinent that some cell lines (already having undergone EMT) are not as sensitive to the biophysical features of their microenvironment.
Keywords: 3D model; EMT; collagen; colorectal cancer; hypoxia; tumour microenvironment; tumour stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
3D collagen fibrillar microstructure guides pancreatic cancer cell phenotype and serves as a critical design parameter for phenotypic models of EMT.PLoS One. 2017 Nov 30;12(11):e0188870. doi: 10.1371/journal.pone.0188870. eCollection 2017. PLoS One. 2017. PMID: 29190794 Free PMC article.
-
Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer.World J Gastroenterol. 2015 Sep 14;21(34):9887-99. doi: 10.3748/wjg.v21.i34.9887. World J Gastroenterol. 2015. PMID: 26379394 Free PMC article.
-
Inhibitory effect of melatonin on hypoxia-induced vasculogenic mimicry via suppressing epithelial-mesenchymal transition (EMT) in breast cancer stem cells.Eur J Pharmacol. 2020 Aug 15;881:173282. doi: 10.1016/j.ejphar.2020.173282. Epub 2020 Jun 21. Eur J Pharmacol. 2020. PMID: 32580038
-
Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics.Semin Cancer Biol. 2023 Jan;88:46-66. doi: 10.1016/j.semcancer.2022.12.002. Epub 2022 Dec 13. Semin Cancer Biol. 2023. PMID: 36521737 Free PMC article. Review.
-
The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment.Biomed Pharmacother. 2016 May;80:393-405. doi: 10.1016/j.biopha.2016.02.044. Epub 2016 Apr 17. Biomed Pharmacother. 2016. PMID: 27133080 Review.
Cited by
-
Patient-specific colorectal-cancer-associated fibroblasts modulate tumor microenvironment mechanics.iScience. 2024 May 21;27(6):110060. doi: 10.1016/j.isci.2024.110060. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883829 Free PMC article.
-
State-of-the-Art Molecular Oncology in UK.Int J Mol Sci. 2023 May 26;24(11):9336. doi: 10.3390/ijms24119336. Int J Mol Sci. 2023. PMID: 37298288 Free PMC article.
-
Cellular Imaging and Time-Domain FLIM Studies of Meso-Tetraphenylporphine Disulfonate as a Photosensitising Agent in 2D and 3D Models.Int J Mol Sci. 2024 Apr 11;25(8):4222. doi: 10.3390/ijms25084222. Int J Mol Sci. 2024. PMID: 38673807 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

