Cell Type-Specific Anti-Viral Effects of Novel SARS-CoV-2 Main Protease Inhibitors
- PMID: 36835380
- PMCID: PMC9959602
- DOI: 10.3390/ijms24043972
Cell Type-Specific Anti-Viral Effects of Novel SARS-CoV-2 Main Protease Inhibitors
Abstract
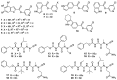
Recently, we have described novel pyridyl indole esters and peptidomimetics as potent inhibitors of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) main protease. Here, we analysed the impact of these compounds on viral replication. It has been shown that some antivirals against SARS-CoV-2 act in a cell line-specific way. Thus, the compounds were tested in Vero, Huh-7, and Calu-3 cells. We showed that the protease inhibitors at 30 µM suppress viral replication by up to 5 orders of magnitude in Huh-7 cells, while in Calu-3 cells, suppression by 2 orders of magnitude was achieved. Three pyridin-3-yl indole-carboxylates inhibited viral replication in all cell lines, indicating that they might repress viral replication in human tissue as well. Thus, we investigated three compounds in human precision-cut lung slices and observed donor-dependent antiviral activity in this patient-near system. Our results provide evidence that even direct-acting antivirals may act in a cell line-specific manner.
Keywords: SARS-CoV-2; azapeptide nitriles; cell line specificity pyridyl indole carboxylates; peptidomimetics; protease inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kemnic T.R., Gulick P.G. StatPearls. StatPearls Publishing, Treasure Island; Tampa, FL, USA: 2022. HIV Antiretroviral Therapy. - PubMed
-
- Breidenbach J., Lemke C., Pillaiyar T., Schäkel L., Al Hamwi G., Diett M., Gedschold R., Geiger N., Lopez V., Mirza S., et al. Targeting the main protease of SARS-CoV-2: From the establishment of high throughput screening to the design of tailored inhibitors. Angew. Chem. Int. Ed. Engl. 2021;60:10423–10429. doi: 10.1002/anie.202016961. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

