Approaches in Hydroxytyrosol Supplementation on Epithelial-Mesenchymal Transition in TGFβ1-Induced Human Respiratory Epithelial Cells
- PMID: 36835384
- PMCID: PMC9967984
- DOI: 10.3390/ijms24043974
Approaches in Hydroxytyrosol Supplementation on Epithelial-Mesenchymal Transition in TGFβ1-Induced Human Respiratory Epithelial Cells
Abstract
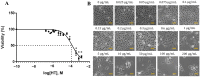
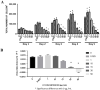
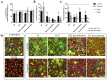
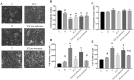
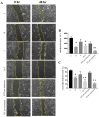
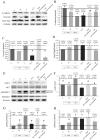
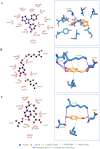
Hydroxytyrosol (HT) is an olive polyphenol with anti-inflammatory and antioxidant properties. This study aimed to investigate the effect of HT treatment on epithelial-mesenchymal transition (EMT) in primary human respiratory epithelial cells (RECs) isolated from human nasal turbinate. HT dose-response study and growth kinetic study on RECs was performed. Several approaches on HT treatment and TGFβ1 induction with varying durations and methods was studied. RECs morphology and migration ability were evaluated. Vimentin and E-cadherin immunofluorescence staining and Western blotting [E-cadherin, vimentin, SNAIL/SLUG, AKT, phosphorylated (p)AKT, SMAD2/3 and pSMAD2/3] were performed after 72-h treatment. In silico analysis (molecular docking) of HT was performed to evaluate the potential of HT to bind with the TGFβ receptor. The viability of the HT-treated RECs was concentration-dependent, where the median effective concentration (EC50) was 19.04 μg/mL. Testing of the effects of 1 and 10 µg/mL HT revealed that HT suppressed expression of the protein markers vimentin and SNAIL/SLUG while preserving E-cadherin protein expression. Supplementation with HT protected against SMAD and AKT pathway activation in the TGFβ1-induced RECs. Furthermore, HT demonstrated the potential to bind with ALK5 (a TGFβ receptor component) in comparison to oleuropein. TGFβ1-induced EMT in RECs and HT exerted a positive effect in modulating the effects of EMT.
Keywords: EMT; fibrosis; inflammation; olive; rhinosinusitis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

Similar articles
-
The potential of Olea europaea extracts to prevent TGFβ1-induced epithelial to mesenchymal transition in human nasal respiratory epithelial cells.BMC Complement Altern Med. 2018 Jun 26;18(1):197. doi: 10.1186/s12906-018-2250-5. BMC Complement Altern Med. 2018. PMID: 29940929 Free PMC article.
-
Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling.Cell Biol Toxicol. 2019 Apr;35(2):95-109. doi: 10.1007/s10565-018-9438-9. Epub 2018 Jul 6. Cell Biol Toxicol. 2019. PMID: 29978330
-
Saponins from the roots of Platycodon grandiflorum suppresses TGFβ1-induced epithelial-mesenchymal transition via repression of PI3K/Akt, ERK1/2 and Smad2/3 pathway in human lung carcinoma A549 cells.Nutr Cancer. 2014;66(1):140-51. doi: 10.1080/01635581.2014.853087. Epub 2013 Dec 16. Nutr Cancer. 2014. PMID: 24341702
-
Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/β-catenin and TGFβ signaling pathways.Eur J Nutr. 2019 Dec;58(8):3207-3219. doi: 10.1007/s00394-018-1864-1. Epub 2018 Nov 21. Eur J Nutr. 2019. PMID: 30460610
-
Dasatinib Suppresses TGFβ-Mediated Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells and Inhibits Pulmonary Fibrosis.Lung. 2018 Oct;196(5):531-541. doi: 10.1007/s00408-018-0134-6. Epub 2018 Jun 20. Lung. 2018. PMID: 29926178
Cited by
-
Hydroxytyrosol: A Promising Therapeutic Agent for Mitigating Inflammation and Apoptosis.Pharmaceutics. 2024 Nov 22;16(12):1504. doi: 10.3390/pharmaceutics16121504. Pharmaceutics. 2024. PMID: 39771483 Free PMC article. Review.
-
Role of Olive Bioactive Compounds in Respiratory Diseases.Antioxidants (Basel). 2023 May 23;12(6):1140. doi: 10.3390/antiox12061140. Antioxidants (Basel). 2023. PMID: 37371870 Free PMC article. Review.
-
Maximizing Postoperative Recovery: The Role of Functional Biomaterials as Nasal Packs-A Comprehensive Systematic Review without Meta-Analysis (SWiM).Pharmaceutics. 2023 May 18;15(5):1534. doi: 10.3390/pharmaceutics15051534. Pharmaceutics. 2023. PMID: 37242776 Free PMC article. Review.
-
An Antibody of the Secreted Isoform of Disintegrin and Metalloprotease 9 (sADAM9) Inhibits Epithelial-Mesenchymal Transition and Migration of Prostate Cancer Cell Lines.Int J Mol Sci. 2024 Jun 17;25(12):6646. doi: 10.3390/ijms25126646. Int J Mol Sci. 2024. PMID: 38928352 Free PMC article.
References
-
- Beatrice M., John A., Ahmed L., Peter M. Prevalence of Chronic Rhinosinusitis in Children with Dyspepsia—A Cross Sectional Study. Egypt. J. Ear Nose Throat Allied Sci. 2016;17:139–142. doi: 10.1016/j.ejenta.2016.07.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

