New Approaches in Early-Stage NSCL Management: Potential Use of PARP Inhibitors and Immunotherapy Combination
- PMID: 36835456
- PMCID: PMC9961654
- DOI: 10.3390/ijms24044044
New Approaches in Early-Stage NSCL Management: Potential Use of PARP Inhibitors and Immunotherapy Combination
Abstract
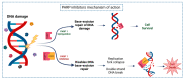
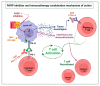
Lung cancer is the second most common cancer in the world, being the first cause of cancer-related mortality. Surgery remains the only potentially curative treatment for Non-Small Cell Lung Cancer (NSCLC), but the recurrence risk remains high (30-55%) and Overall Survival (OS) is still lower than desirable (63% at 5 years), even with adjuvant treatment. Neoadjuvant treatment can be helpful and new therapies and pharmacologic associations are being studied. Immune Checkpoint Inhibitors (ICI) and PARP inhibitors (PARPi) are two pharmacological classes already in use to treat several cancers. Some pre-clinical studies have shown that its association can be synergic and this is being studied in different settings. Here, we review the PARPi and ICI strategies in cancer management and the information will be used to develop a clinical trial to evaluate the potential of PARPi association with ICI in early-stage neoadjuvant setting NSCLC.
Keywords: EARLY-stage NSCLC; PARP inhibitors; combination therapy; immunotherapy; neoadjuvant setting; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rolf A., Stahel S.P. In: Thoracic Tumours: Essentials for Clinicians. 2nd ed. Marina Chiara Garassino, editor. ESMO Press; Lugano, Switzerland: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

