The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology
- PMID: 36835499
- PMCID: PMC9962350
- DOI: 10.3390/ijms24044089
The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology
Abstract
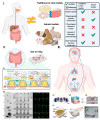
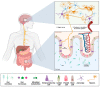
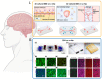
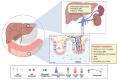
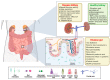
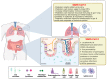
The intestine is considered to be a vital digestive organ to absorb nutrients and is the largest immune organ, while numerous microorganisms coexist with the host. It is well known that the complex interactions between the gut microbiota and the host's immune system inevitably affect the function of other organs, creating an "axis" between them. During the past few years, a new technique based mainly on microfluidics and cell biology has been developed to emulate the structure, function, and microenvironment of the human gut, called the "gut-on-chip". This microfluidic chip provides insight into key aspects of gut function in health and disease, such as the gut-brain axis, gut-liver axis, gut-kidney axis, and gut-lung axis. In this review, we first describe the basic theory of the gut axis and the various composition and parameter monitoring of the gut microarray systems, as well as summarize the development and emerging advances in the gut-organ-on-chip, with a focus on the host-gut flora and nutrient metabolism, and highlight their role in pathophysiological studies. In addition, this paper discusses the challenges and prospects for the current development and further use of the gut-organ-on-chip platform.
Keywords: bio-inspired microfluidic; disease; gut–organ-axis; gut–organ-on-chip; organ-on-chip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Grants and funding
- 2021YFD1600400/National Key Research and Development Program
- 2020GY-236/General Plan of Shaanxi Province
- 2021ZDLNY04-01/Key industrial chain projects of Shaanxi Province-Agricultural Field
- 2022ZDLNY04-05/Key industrial chain projects of Shaanxi Province-Agricultural Field
- 202131/Project from Weiyang Technology Bureau
- 21NYYF0022/Project from Xi 'an City innovation plan- Agricultural Field
- ZNGQCX-A-2020003/Project from Ningxia Zhongning Goji Industry Innovation Research Institute
- 2022NY-035/General Plan of Shaanxi Province
- 22JC021/Industrialization projects of the Education Department of Shaanxi Province
- S2022-ZC-QCYK-0011/project from Qinchuang Yuan "Scientist& Engineers" Team
LinkOut - more resources
Full Text Sources
Miscellaneous

