Synthetic Small Molecule Modulators of Hsp70 and Hsp40 Chaperones as Promising Anticancer Agents
- PMID: 36835501
- PMCID: PMC9964478
- DOI: 10.3390/ijms24044083
Synthetic Small Molecule Modulators of Hsp70 and Hsp40 Chaperones as Promising Anticancer Agents
Abstract
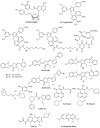
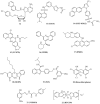
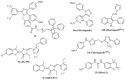
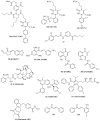
A class of chaperones dubbed heat shock protein 70 (Hsp70) possesses high relevance in cancer diseases due to its cooperative activity with the well-established anticancer target Hsp90. However, Hsp70 is closely connected with a smaller heat shock protein, Hsp40, forming a formidable Hsp70-Hsp40 axis in various cancers, which serves as a suitable target for anticancer drug design. This review summarizes the current state and the recent developments in the field of (semi-)synthetic small molecule inhibitors directed against Hsp70 and Hsp40. The medicinal chemistry and anticancer potential of pertinent inhibitors are discussed. Since Hsp90 inhibitors have entered clinical trials but have exhibited severe adverse effects and drug resistance formation, potent Hsp70 and Hsp40 inhibitors may play a significant role in overcoming the drawbacks of Hsp90 inhibitors and other approved anticancer drugs.
Keywords: Hsp40; Hsp70; anticancer agents; heat shock proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

