Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management
- PMID: 36835512
- PMCID: PMC9960633
- DOI: 10.3390/ijms24044091
Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management
Abstract
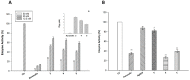
Bis(pyrazol-1-yl)acetic acid (HC(pz)2COOH) and bis(3,5-dimethyl-pyrazol-1-yl)acetic acid (HC(pzMe2)2COOH) were converted into the methyl ester derivatives 1 (LOMe) and 2 (L2OMe), respectively, and were used for the preparation of silver(I) complexes 3-5. The Ag(I) complexes were prepared by the reaction of AgNO3 and 1,3,5-triaza-7-phosphaadamantane (PTA) or triphenylphosphine (PPh3) with LOMe and L2OMe in methanol solution. All Ag(I) complexes showed a significant in vitro antitumor activity, proving to be more effective than the reference drug cisplatin in the in-house human cancer cell line panel containing examples of different solid tumors. Compounds were particularly effective against the highly aggressive and intrinsically resistant human small-cell lung carcinoma (SCLC) cells, either in 2D and 3D cancer cell models. Mechanistic studies revealed their ability to accumulate into cancer cells and to selectively target Thioredoxin (TrxR), thus leading to redox homeostasis unbalance and ultimately inducing cancer cell death through apoptosis.
Keywords: TrxR; anticancer activity; bis(pyrazolyl)acetate ligands; oxidative stress; silver.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Anticancer potential of copper(i) complexes based on isopropyl ester derivatives of bis(pyrazol-1-yl)acetate ligands.RSC Med Chem. 2024 Nov 13;16(2):849-861. doi: 10.1039/d4md00610k. eCollection 2025 Feb 19. RSC Med Chem. 2024. PMID: 39618961 Free PMC article.
-
Exploring the Antitumor Potential of Copper Complexes Based on Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands.Int J Mol Sci. 2022 Aug 20;23(16):9397. doi: 10.3390/ijms23169397. Int J Mol Sci. 2022. PMID: 36012662 Free PMC article.
-
Syntheses and Biological Studies of Cu(II) Complexes Bearing Bis(pyrazol-1-yl)- and Bis(triazol-1-yl)-acetato Heteroscorpionate Ligands.Molecules. 2019 May 7;24(9):1761. doi: 10.3390/molecules24091761. Molecules. 2019. PMID: 31067640 Free PMC article.
-
Synthesis and Cytotoxic Activity Evaluation of New Cu(I) Complexes of Bis(pyrazol-1-yl) Acetate Ligands Functionalized with an NMDA Receptor Antagonist.Int J Mol Sci. 2020 Apr 9;21(7):2616. doi: 10.3390/ijms21072616. Int J Mol Sci. 2020. PMID: 32283777 Free PMC article.
-
Antiproliferative Homoleptic and Heteroleptic Phosphino Silver(I) Complexes: Effect of Ligand Combination on Their Biological Mechanism of Action.Molecules. 2020 Nov 23;25(22):5484. doi: 10.3390/molecules25225484. Molecules. 2020. PMID: 33238608 Free PMC article.
Cited by
-
Synthesis and Investigations of the Antitumor Effects of First-Row Transition Metal(II) Complexes Supported by Two Fluorinated and Non-Fluorinated β-Diketonates.Int J Mol Sci. 2024 Feb 7;25(4):2038. doi: 10.3390/ijms25042038. Int J Mol Sci. 2024. PMID: 38396717 Free PMC article.
-
Silver and Copper Complexes with Ibuprofen and Caffeine-Preparation and Evaluation of Their Selected Biological Effects.Molecules. 2024 Jan 19;29(2):506. doi: 10.3390/molecules29020506. Molecules. 2024. PMID: 38276584 Free PMC article.
-
Anticancer potential of copper(i) complexes based on isopropyl ester derivatives of bis(pyrazol-1-yl)acetate ligands.RSC Med Chem. 2024 Nov 13;16(2):849-861. doi: 10.1039/d4md00610k. eCollection 2025 Feb 19. RSC Med Chem. 2024. PMID: 39618961 Free PMC article.
-
The Elusive Biological Activity of Scorpionates: A Useful Scaffold for Cancer Therapy?Molecules. 2024 Nov 30;29(23):5672. doi: 10.3390/molecules29235672. Molecules. 2024. PMID: 39683831 Free PMC article. Review.
-
New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity.Molecules. 2025 Apr 24;30(9):1893. doi: 10.3390/molecules30091893. Molecules. 2025. PMID: 40363700 Free PMC article.
References
-
- Medici S., Peana M., Crisponi G., Nurchi V.M., Lachowicz J.I., Remelli M., Zoroddu M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016;327–328:349–359. doi: 10.1016/j.ccr.2016.05.015. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

