Development of Specific Monoclonal Antibodies against Porcine RIG-I-like Receptors Revealed the Species Specificity
- PMID: 36835527
- PMCID: PMC9967608
- DOI: 10.3390/ijms24044118
Development of Specific Monoclonal Antibodies against Porcine RIG-I-like Receptors Revealed the Species Specificity
Abstract
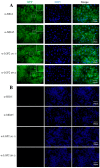
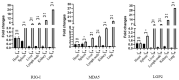
The RIG-I-like receptors (RLRs) play critical roles in sensing and combating viral infections, particularly RNA virus infections. However, there is a dearth of research on livestock RLRs due to a lack of specific antibodies. In this study, we purified porcine RLR proteins and developed monoclonal antibodies (mAbs) against porcine RLR members RIG-I, MDA5 and LGP2, for which one, one and two hybridomas were obtained, respectively. The porcine RIG-I and MDA5 mAbs each targeted the regions beyond the N-terminal CARDs domains, whereas the two LGP2 mAbs were both directed to the N-terminal helicase ATP binding domain in the Western blotting. In addition, all of the porcine RLR mAbs recognized the corresponding cytoplasmic RLR proteins in the immunofluorescence and immunochemistry assays. Importantly, both RIG-I and MDA5 mAbs are porcine specific, without demonstrating any cross-reactions with the human counterparts. As for the two LGP2 mAbs, one is porcine specific, whereas another one reacts with both porcine and human LGP2. Thus, our study not only provides useful tools for porcine RLR antiviral signaling research, but also reveals the porcine species specificity, giving significant insights into porcine innate immunity and immune biology.
Keywords: RLRs; human; monoclonal antibody; porcine; species specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Analysis of Porcine RIG-I Like Receptors Revealed the Positive Regulation of RIG-I and MDA5 by LGP2.Front Immunol. 2021 May 18;12:609543. doi: 10.3389/fimmu.2021.609543. eCollection 2021. Front Immunol. 2021. PMID: 34093517 Free PMC article.
-
LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses.Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1512-7. doi: 10.1073/pnas.0912986107. Epub 2010 Jan 8. Proc Natl Acad Sci U S A. 2010. PMID: 20080593 Free PMC article.
-
RIG-I-like receptors: Molecular mechanism of activation and signaling.Adv Immunol. 2023;158:1-74. doi: 10.1016/bs.ai.2023.03.001. Epub 2023 May 9. Adv Immunol. 2023. PMID: 37453753 Review.
-
Paramyxovirus V protein interaction with the antiviral sensor LGP2 disrupts MDA5 signaling enhancement but is not relevant to LGP2-mediated RLR signaling inhibition.J Virol. 2014 Jul;88(14):8180-8. doi: 10.1128/JVI.00737-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829334 Free PMC article.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
Cited by
-
The Novel Antigenic Epitopes of African Swine Fever Virus Inner Membrane p54 Protein Revealed by Monoclonal Antibodies.Animals (Basel). 2025 Apr 30;15(9):1296. doi: 10.3390/ani15091296. Animals (Basel). 2025. PMID: 40362108 Free PMC article.
-
Specific Monoclonal Antibodies against African Swine Fever Virus Protease pS273R Revealed a Novel and Conserved Antigenic Epitope.Int J Mol Sci. 2024 Aug 15;25(16):8906. doi: 10.3390/ijms25168906. Int J Mol Sci. 2024. PMID: 39201592 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

