Application of Gold Nanoparticles as Radiosensitizer for Metastatic Prostate Cancer Cell Lines
- PMID: 36835538
- PMCID: PMC9964626
- DOI: 10.3390/ijms24044122
Application of Gold Nanoparticles as Radiosensitizer for Metastatic Prostate Cancer Cell Lines
Abstract
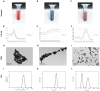
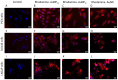
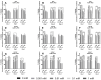
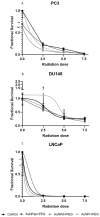
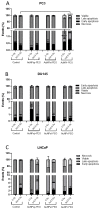
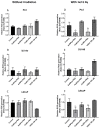
More than 50% of all prostate cancer (PCa) patients are treated by radiotherapy (RT). Radioresistance and cancer recurrence are two consequences of the therapy and are related to dose heterogeneity and non-selectivity between normal and tumoral cells. Gold nanoparticles (AuNPs) could be used as potential radiosensitizers to overcome these therapeutic limitations of RT. This study assessed the biological interaction of different morphologies of AuNPs with ionizing radiation (IR) in PCa cells. To achieve that aim, three different amine-pegylated AuNPs were synthesized with distinct sizes and shapes (spherical, AuNPsp-PEG, star, AuNPst-PEG, and rods, AuNPr-PEG) and viability, injury and colony assays were used to analyze their biological effect on PCa cells (PC3, DU145, and LNCaP) when submitted to the accumulative fraction of RT. The combinatory effect of AuNPs with IR decreased cell viability and increased apoptosis compared to cells treated only with IR or untreated cells. Additionally, our results showed an increase in the sensitization enhancement ratio by cells treated with AuNPs and IR, and this effect is cell line dependent. Our findings support that the design of AuNPs modulated their cellular behavior and suggested that AuNPs could improve the RT efficacy in PCa cells.
Keywords: gold nanoparticles; in vitro assay; prostate cancer; radiosensitizing effect; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



Similar articles
-
Radiotherapy Metastatic Prostate Cancer Cell Lines Treated with Gold Nanorods Modulate miRNA Signatures.Int J Mol Sci. 2024 Feb 27;25(5):2754. doi: 10.3390/ijms25052754. Int J Mol Sci. 2024. PMID: 38474003 Free PMC article.
-
Metabolic Disruption of Gold Nanospheres, Nanostars and Nanorods in Human Metastatic Prostate Cancer Cells.Cells. 2023 Mar 2;12(5):787. doi: 10.3390/cells12050787. Cells. 2023. PMID: 36899923 Free PMC article.
-
Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles.Int J Mol Sci. 2022 May 9;23(9):5279. doi: 10.3390/ijms23095279. Int J Mol Sci. 2022. PMID: 35563666 Free PMC article.
-
Radiosensitization by gold nanoparticles: Will they ever make it to the clinic?Radiother Oncol. 2017 Sep;124(3):344-356. doi: 10.1016/j.radonc.2017.07.007. Epub 2017 Aug 4. Radiother Oncol. 2017. PMID: 28784439 Review.
-
Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy.Int J Nanomedicine. 2020 Nov 24;15:9407-9430. doi: 10.2147/IJN.S272902. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33262595 Free PMC article. Review.
Cited by
-
A Step Forward for the Treatment of Localized Prostate Cancer Using Gold Nanoparticles Combined with Laser Irradiation.Int J Mol Sci. 2024 Apr 19;25(8):4488. doi: 10.3390/ijms25084488. Int J Mol Sci. 2024. PMID: 38674073 Free PMC article.
-
Microdosimetric Simulation of Gold-Nanoparticle-Enhanced Radiotherapy.Int J Mol Sci. 2024 Sep 2;25(17):9525. doi: 10.3390/ijms25179525. Int J Mol Sci. 2024. PMID: 39273472 Free PMC article.
-
In vitro study of effect of gold nanoparticles conjugated with triptorelin peptide on the radiosensitivity of breast cancer cells (MCF-7).Discov Oncol. 2025 Mar 26;16(1):404. doi: 10.1007/s12672-025-01935-3. Discov Oncol. 2025. PMID: 40140152 Free PMC article.
-
Do cell culturing influence the radiosensitizing effect of gold nanoparticles: a Monte Carlo study.EJNMMI Phys. 2025 Apr 18;12(1):41. doi: 10.1186/s40658-025-00746-3. EJNMMI Phys. 2025. PMID: 40249448 Free PMC article.
-
Radiotherapy Metastatic Prostate Cancer Cell Lines Treated with Gold Nanorods Modulate miRNA Signatures.Int J Mol Sci. 2024 Feb 27;25(5):2754. doi: 10.3390/ijms25052754. Int J Mol Sci. 2024. PMID: 38474003 Free PMC article.
References
-
- Perez C., Halperin E., Brady L. Principles and Practice of Radiation Oncology 5ª EDIÇÃO. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2008.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

